Abstract
A defective production of protective levels of antibodies to Hepatitis B (HB) vaccine is reported to occur in 4–10% of healthy subjects and a correlation with the presence of specific human leukocyte antigen (HLA) molecules, including DQ2, which also confers genetic predisposition to celiac disease (CD) and type I diabetes mellitus (T1DM), has been suggested.
The aim of this study was to analyze the serological response to HB vaccine and measles-containing vaccines in 69 diabetic patients (T1DM), 42 patients with celiac disease (CD) and 79 healthy control subjects (CT).
The median interval between the third dose of HB vaccine and serum collection was 6.8, 3.5, and 4.7 years for T1DM, CD and CT groups, respectively. 50/69 (72%) T1DM patients, 32/42 (76%) CD patients and 61/79 (77%) CT subjects showed protective anti-HBs antibodies after vaccination, with no statistically significant difference. On the contrary, a lower statistically significant difference was found in the mean HBsAb level of T1DM subjects when compared with the other two groups. No correlation between HLA DQ2 expression in T1DM and vaccine response was detected.
The comparison of serological response to measles after vaccination also showed no statistically significant differences in the three groups.
Contrasting results between these data and those reported in the literature might be due to differences in the time intervals between vaccination and testing.
Prospective studies in pathological and healthy groups with the same age at HBV vaccination and with the same time interval for blood sample collection to determine antibody titers are necessary in order to provide more conclusive data.
Introduction
In order to reduce the complications related to the worldwide diffuse hepatitis B virus (HBV) infection, mainly cirrhosis and hepatocarcinoma, HBV vaccine was introduced in the early 1980s.
Initially applied to people at high risk for HBV infection, the vaccine is currently administered to all infants in many countries. It has been demonstrated that this vaccine confers long-term protection and immune memory to HBsAg beyond the time at which protective anti-HBs antibodies disappear.Citation1
A lack of or a low response to vaccination is reported to occur between 4% and 10% of healthy subjects.Citation2 A defective response has been correlated with age, smoking, obesity, male genderCitation3 and is also reported in carriers of specific human leukocyte antigen (HLA) molecules, including DQ2, which is expressed in the large majority of celiac (CD) and type 1 diabetic (T1DM) patients; this genetic feature is believed to be the main cause of the lower anti-HBs antibody titer after vaccinationCitation4 assessed in CD and T1DM subjects than in the control healthy group (CT), as previously reported.5,6. In regard to HB vaccine efficacy in pathological subjects, most studies evaluated seroconversion rates in hemodialyzed patients, including diabetics, but this condition per se is related to specific issues and defective response.Citation7
Measles vaccines are very effective in eliciting antibody responses; seroconversion is reported in about 95% of healthy subjects. Besides vaccine characteristics, immunogenicity is also known to be influenced by genetic factors including HLA alleles.Citation8 To our knowledge data on immune response to measles vaccine in subjects with CD or T1DM have not been reported yet, while specific antibody positivity to other antigens included in pediatric vaccines has been found in CD children.Citation5
The aim of this study was to analyze the serological response to HBV and measles-containing vaccines in a group of diabetic, a group of celiac patients and a control group in order to clarify the influence of these underlying immuno-mediated diseases on vaccine immune response.
Patients and Methods
Patients
The study population consists of 69 consecutive patients affected by T1DM, referred to the Regional Center for Pediatric Diabetes of Verona, who were periodically followed up. Sixty-six/69 (96.5%) diabetic patients included in this retrospective study had received the HB vaccination (3 doses) in the first year of age, according to the national vaccination schedule; two were vaccinated at 11 y and one at 5 y of age. They were also HLA-typed for DQ2 and DQ8 alleles by PCR amplification of genomic DNA by high resolution HLA-DQA1 and -DQB1 typing kits (Thermo Fisher Scientific Inc, USA).
Sixty-four/69 (92.7%) T1DM patients were also vaccinated against measles by receiving the trivalent measles-mumps-rubella (MMR) vaccine; 33/64 (51.5%) received 1 dose at 12–24 months of age and were considered for analysis.
Forty-two consecutive CD patients with documented HB vaccination were selected as a further population for this retrospective study. They had also received HB vaccination in the first year of age. Only 3/42 (7%) were already on gluten free diet at the time of vaccination. Thirty-five/42 (83.3%) CD patients were also vaccinated against measles by receiving the trivalent MMR vaccine; 28/35 (80%) received 1 dose, 27 at 12–24 mo of age and one at 18 years of age. Only 1/28 (3.4%) CD patient was on a gluten-free diet at the time of vaccination.
Seventy-nine consecutive CT subjects, negative for CD and T1DM autoantibody markers and with documented HB vaccination according to the national schedule, were selected as a control group. Fifty-five/79 (69.6%) CT subjects were also vaccinated against measles by receiving the trivalent MMR vaccine. Forty-three/55 (78.1%) of CT subjects were vaccinated with 1 dose of MMR vaccine; 39 at 12–24 months of age, four at 3, 4, 5, 13 years of age respectively.
A second dose of MMR vaccine was administered to 31/64 (48.4%) of T1DM patients, to 7/35 (0.2%) of CD patients, 12/55 (22%) CT subjects at 6 or 12 years of age. Since not all subjects received a second dose of MMR vaccine, only those who received one dose were considered for analysis.
The median intervals between the third dose of HB vaccine or measles-containing vaccine and serum collection are reported in . The brand of vaccines administered were not reported in the vaccination records. HLA typing was performed only in T1DM patients for reasons of costs.
Table 1. Characteristics of the study population
Subjects with other autoimmune diseases or an incomplete vaccination schedule were excluded.
All subjects or their parents or guardians gave informed consent to the management of their clinical data and serum analysis for research purposes, as per national privacy laws.
Methods
Specific HB surface antibody (HBsAb) determination was performed by the commercially available ELISA kit ETI-AB-AUK-3 (Diasorin SpA, Vercelli, Italy) with a cut-off value for protection of HBsAb of 10 IU/L. Antibody levels were distributed into 4 ranges: 0, 1–9, 10–100, >100 IU/L. Quantitative data were also analyzed.
Quantification of specific IgG to measles virus was performed with the NovaLisa Measles Virus kit (NovaTec Immundiagnostica, Dietzenbach, Germany), as per manufacturer's instructions; with a cut off value for positivity of 11 NovaTec Units (NTU), considering border line results of 9–11 NTU as positive in the analysis.
Statistical analysis
Anti-HBs titer underwent log10 transformation in order to obtain better (more normal) distribution. An analysis of the variance (ANOVA) model was performed, using the clinical groups (T1DM, CD and controls) as the categorical predictor. The means of the various groups were then contrasted, in order to obtain a series of paired comparisons.
Since the three groups were enrolled in different clinical settings, average effect size of risk due to T1DM and CD was evaluated with a procedure that estimates average treatment effects (TE) from observational data via regression adjustment (RA). The dependent variable (outcome) was log10 anti-HBs concentration, “treatment” was the clinical condition (either T1DM or CD) compared with controls, and possible controlled confounders were gender and age.
All estimations were calculated with Stata 13.1.
Results
Data regarding the age at sample collection and time interval between vaccination and serological testing for T1DM, CD, and controls subjects are reported in , as well as HLA typing in T1DM patients. Sex and median age of patients and controls at the time of testing did not differ statistically between the 3 groups.
Results of serological testing are summarized in . The overall proportion of subjects showing protective level of HBsAb did not differ in the three groups analyzed. However, a statistically significant difference was found in the mean HBsAb levels, which resulted lower in T1DM subjects when compared with the other two groups. A correlation between the presence of HLA DQ2 and defective serological protection against HB was not found in our study population of T1DM patients.
Table 2. Specific antibody levels against HBV and measles in patients and controls
In regard to the persistency of serological protection against measles after 1 dose of vaccine in the three groups of subjects, we found positive anti-measles antibody levels in 88% of T1DM patients, 75% of CD patients and 89% of controls, with no statistically significant differences.
Similar anti-measles antibody concentrations in the three study groups were found as well. A possible role of HLA DQ2 in hyporesponsiveness against measles vaccine was not found in T1DM patients.
shows the means and SD of the anti-HBs titer in the three clinical groups under investigation. The log10 transformed anti-HBs titer is shown in . The clinical groups appeared to differ significantly regarding the anti-HBs titer under log10 transformation (R-squared = 0.0520, F = 5.13, P = 0.0068), thus suggesting that the groups responded to HB vaccine in a different way. The paired contrasts between the various groups are reported in . They demonstrate that the anti-HBs titer in T1DM was significantly lower than in controls (P = 0.004) and in CD (P = 0.013). Instead, no significant difference was detected between controls and CD patients. The TE procedure revealed a significant lowering effect on antibody production after HB vaccine administration, measured as log10 anti-HBs titer, due to T1DM. On the other hand, the effect of CD was not significant (indeed absent).
Figure 1. Log10- transformed anti-HBs titer, by clinical groups. Means and 95% confidence intervals of the means are indicated. CT, control subjects; T1DM, type I diabetes mellitus patients; CD, celiac disease patients.
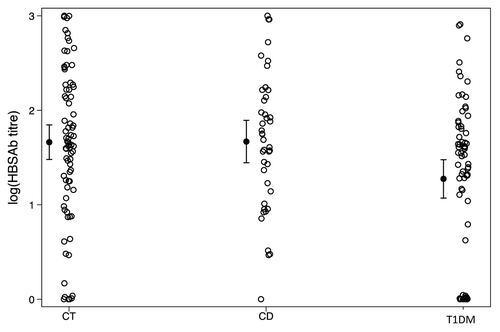
Table 3. Log10- transformed anti-HBs titer: Pairwise contrasts between the three clinical groups, CT, T1DM, CD
Discussion
In this study we retrospectively analyzed the response to HBV and measles vaccine in a group of T1DM and CD patients, comparing the results with those obtained from a healthy control group.
HBV vaccine elicits a protective immune response in about 95% healthy individuals,Citation9 although lower frequencies of seroconversion (75%) have been reported by other authors.Citation10 Non-responsiveness to HBV vaccine has been associated with factors such as chronic diseases, smoking, obesity and the male gender.Citation3 A genetic predisposition to HBV vaccine non-responsiveness has been attributed to particular HLA antigens,Citation11,Citation12 mainly DQ2 haplotype, which is also involved in autoimmunity.Citation13 In fact, while DQ2 is present in nearly 40% of the general population, it is expressed in 81% of CD patients. DQ8 is expressed in 5–8% of CD cases. CD shares common HLA alleles with diabetes mellitus since approximately 90% of T1DM patients express DQ2 or DQ8.Citation4,Citation14 Unresponsiveness or low response to HBV vaccine are reported to occur in about 50% of T1DM or CD patientsCitation5,Citation6; as a consequence, it was hypothesized that HBsAg presentation may occur with low affinity by DQ2 in these genetically predisposed individuals.Citation11
Besides this genetic background, the titer of anti-HBV antibodies also depends on age at vaccinationCitation3 and declines in time, that may be faster in T1DM and CD patients, until it becomes undetectable in 15–50% cases within 5 to 10 years.Citation15 Therefore, the level of antibodies is related to the time interval between vaccination and blood sample collection for testing.
Here we tried to establish whether responses to HBV vaccine and levels of serum antibodies in a group of T1DM, in a group of CD patients and in a CT group were also influenced by the time interval between vaccination and collection of blood samples; in T1DM patients a possible relation between DQ2 expression and unresponsiveness was evaluated.
Furthermore, we analyzed the effect of measles vaccination in the same T1DM and CD patients; although a normal response after tetanus, rubella, and Hemophilus influenzae type b vaccines was reported by other authorsCitation5 in a group of CD children, to our knowledge, no data are available in the literature regarding the efficacy of measles vaccine in T1DM and CD patients.
On the whole, we detected no significant differences in the percentage of responders to HBV and measles vaccines among T1DM, CD patients and the control group.
In T1DM patients, however, a statistically significant lower mean concentration of anti-HBV antibodies was found than in CD patients and in the control group, but no correlation between DQ2 haplotype and responsiveness was detected. A lack of correlation between HBV vaccine response and DQ2 expression is reported also by another author.10.
Moreover, for CD subjects, at the time of vaccination the large majority of our patients were not on a gluten-free diet, in spite of the fact that gluten-containing diet is believed to be related to unresponsiveness.Citation12
Furthermore an inverse correlation between levels of anti-HBs antibodies and the lag interval from vaccination and sample collection for testing might exist and, almost in part, explain the different results reported in the literature and represent a confounding factor in the evaluation of results. In fact, a prospective study, with the same lag time between vaccine and testing, described a failure to respond to HBV vaccine in CD patients on gluten-free diet in a percentage similar to that observed in healthy people.Citation10 Once again, the hypothesis of immune hyporesponsiveness is not sustainable considering that a high percentage of responders to a booster dose of HBV vaccine is reported in previously vaccinated CD patients without protective anti HBs antibodies.Citation10 In this case, as well, blood testing was performed in all the patients with the same time interval, a short period after vaccination.
As regards the response to measles vaccine, only subjects who received one dose of vaccine were evaluated. A lower percentage of responders in CD patients was detected, but the difference was not statistically significant when compared with the other two groups.
In our opinion, the conflicting results between our findings and the data reported in the literature may be due to differences in ages at vaccination of examined subjects and the differences in time intervals between vaccination and blood sample collection for testing.
Prospective studies in pathological and healthy groups with the same age at HBV vaccination and with the same time interval for blood sample collection to determine antibody levels are necessary in order to provide more conclusive data and to see if it is necessary to revise the vaccination schedule in these categories of patients.
| Abbreviations: | ||
| HB | = | hepatitis B |
| HBsAb | = | antibodies to Hepatitis B surface antigen |
| trivalent measles-mumps-rubella | = | MMR, T1DM, type I diabetes mellitus |
| CD | = | celiac disease |
| CT | = | control subjects |
| HLA | = | human leucocyte antigen |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was financially supported by the Regional Public Health Authority. The authors would like to thank Dr Fabiola Micheletti and Dr Mario Cruciani for collaboration, Ms Elisa Boccola for technical assistance, and Prof Mark J Newman for manuscript revision.
References
- SpadaE, RomanòL, TostiME, ZuccaroO, PaladiniS, ChironnaM, CoppolaRC, CucciaM, MangioneR, MarroneF, et al, the Study Group. Hepatitis B immunity in teenagers vaccinated as infants: an Italian 17-year follow-up study. Clin Microbiol Infect2014; http://dx.doi.org/10.1111/1469-0691.12591; PMID: 24528380
- World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec2009; 84:405 - 19; PMID: 19817017
- ScolnickEM, McLeanAA, WestDJ, McAleerWJ, MillerWJ, BuynakEB. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA1984; 251:2812 - 5; http://dx.doi.org/10.1001/jama.1984.03340450028021; PMID: 6232402
- SjogrenMH. Prevention of hepatitis B in nonresponders to initial hepatitis B virus vaccination. Am J Med2005; 118:Suppl 10A34S - 9S; http://dx.doi.org/10.1016/j.amjmed.2005.07.012; PMID: 16271539
- ParkSD, MarkowitzJ, PetteiM, WeinsteinT, SisonCP, SwissSR, LevineJ. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr2007; 44:431 - 5; http://dx.doi.org/10.1097/MPG.0b013e3180320654; PMID: 17414139
- LeonardiS, VitalitiG, GarozzoMT, Miraglia del GiudiceM, MarsegliaG, La RosaM. Hepatitis B vaccination failure in children with diabetes mellitus? The debate continues. Hum Vaccin Immunother2012; 8:448 - 52; http://dx.doi.org/10.4161/hv.19107; PMID: 22370513
- FabriziF, DixitV, MartinP, MessaP. Meta-analysis: the impact of diabetes mellitus on the immunological response to hepatitis B virus vaccine in dialysis patients. Aliment Pharmacol Ther2011; 33:815 - 21; http://dx.doi.org/10.1111/j.1365-2036.2011.04589.x; PMID: 21281319
- Strebel PM, Papania MJ, Dayan GH, Halsey NA. Measles vaccine. In Plotkin S, Orestein W, Offit P. Vaccines Fifth edition Saunders Ed, 2008:353-398.
- CoatesT, WilsonR, PatrickG, AndréF, WatsonV. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther2001; 23:392 - 403; http://dx.doi.org/10.1016/S0149-2918(01)80044-8; PMID: 11318074
- NemesE, LeflerE, SzegediL, KapitányA, KovácsJB, BaloghM, SzabadosK, TumpekJ, SipkaS, Korponay-SzabóIR. Gluten intake interferes with the humoral immune response to recombinant hepatitis B vaccine in patients with celiac disease. Pediatrics2008; 121:e1570 - 6; http://dx.doi.org/10.1542/peds.2007-2446; PMID: 18519462
- AlperCA, KruskallMS, Marcus-BagleyD, CravenDE, KatzAJ, BrinkSJ, DienstagJL, AwdehZ, YunisEJ. Genetic prediction of nonresponse to hepatitis B vaccine. N Engl J Med1989; 321:708 - 12; http://dx.doi.org/10.1056/NEJM198909143211103; PMID: 2528067
- ZingoneF, MoriscoF, ZanettiA, RomanòL, PortellaG, CaponeP, AndreozziP, TortoraR, CiacciC. Long-term antibody persistence and immune memory to hepatitis B virus in adult celiac patients vaccinated as adolescents. Vaccine2011; 29:1005 - 8; http://dx.doi.org/10.1016/j.vaccine.2010.11.060; PMID: 21129395
- GodkinA, DavenportM, HillAV. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness. Hepatology2005; 41:1383 - 90; http://dx.doi.org/10.1002/hep.20716; PMID: 15915462
- MartinettiM, De SilvestriA, BelloniC, PasiA, TinelliC, PistorioA, SalvaneschiL, RondiniG, AvanziniMA, CucciaM. Humoral response to recombinant hepatitis B virus vaccine at birth: role of HLA and beyond. Clin Immunol2000; 97:234 - 40; http://dx.doi.org/10.1006/clim.2000.4933; PMID: 11112362
- WainwrightRB, BulkowLR, ParkinsonAJ, ZanisC, McMahonBJ. Protection provided by hepatitis B vaccine in a Yupik Eskimo population--results of a 10-year study. J Infect Dis1997; 175:674 - 7; http://dx.doi.org/10.1093/infdis/175.3.674; PMID: 9041341
