Abstract
A trivalent inactivated influenza vaccine (Fluarix™, GlaxoSmithKline Biologicals) was licensed under US accelerated approval regulations. We performed a randomized, observer-blind, post-approval study to demonstrate its immunological non-inferiority versus an established US-licensed vaccine (primary endpoint). Adult (including elderly) subjects received a single injection of newly-licensed vaccine (n=923) or established vaccine (n=922). Serum hemagglutination-inhibition titers were determined pre-vaccination and 21-28 days after vaccination. Non-inferiority was assessed by post-vaccination geometric mean titer (GMT) ratio (upper 95% confidence interval [CI] ≤1.5) and difference in seroconversion rate (upper 95% CI ≤0.1) for all three vaccine strains. Safety was monitored for 6 months. The newly-licensed vaccine was non-inferior to the established vaccine in all subjects (≥18 years) and in elderly subjects (≥65 years). Adjusted GMT ratios (established/newly-licensed) against the H1N1, H3N2 and B strains were 0.65 (95% CI: 0.58, 0.73), 0.93 (0.83, 1.04) and 1.13 (1.03, 1.25) for all subjects and 0.75 (0.67, 0.85), 0.95 (0.82, 1.09) and 1.13 (1.00, 1.27) for elderly subjects. Corresponding values for the differences in seroconversion rate (established minus newly-licensed) were -0.12 (-0.16, -0.07), -0.02 (-0.06, 0.03) and 0.01 (-0.04, 0.06) for all subjects and -0.11 (-0.16, -0.05), -0.02 (-0.07, 0.04) and 0.02 (-0.04, 0.08) for elderly subjects. The most common adverse events with both vaccines were injection site pain, fatigue and headache, and no serious adverse events or deaths were considered related; there were no clinically relevant differences between the vaccines. In conclusion, the newly-licensed vaccine was well tolerated and immunologically non-inferior to the established vaccine for all three vaccine strains in the whole population and the elderly.
Introduction
Influenza is an acute viral disease of the respiratory tract that is estimated to affect 5–15% of the population worldwide, accounting annually for 3–5 million cases of severe illness and 250,000–500,000 deaths.Citation1 In the United States (US) alone, approximately 226,000 hospitalizations and 36,000 deaths occur each year as a result of influenza.Citation2,Citation3 Although influenza affects all age groups, its greatest impact occurs in the elderly, very young children, and people with underlying medical conditions that put them at increased risk of influenza-related complications.Citation3–Citation5 For example, a mortality rate of 98.3 per 100,000 person-years has been reported for people aged ≥65 years in contrast with a rate of only 0.5 per 100,000 person-years for those aged 5–49 years.Citation2 The elderly are also more likely to be hospitalized for influenza.Citation3
Annual vaccination is considered to be the most effective way to reduce the impact of seasonal influenza,Citation6 and vaccine efficacy has been shown to be approximately 80% in healthy adults aged up to 65 years when vaccine strains are well matched to circulating strains.Citation7,Citation8 Variations in disease attack rates and circulating strains, which are difficult to predict in advance can, have a negative impact on vaccine efficacy.Citation9,Citation10 However, a 90% reduction in hospitalization has been reported even when strains are poorly matched.Citation11 Vaccine efficacy in the elderly is lower than is seen in younger adults, largely because of the waning immune response in this population.Citation12 Nevertheless, influenza vaccination has been shown to be effective in prevention of hospitalization and mortality in elderly people.Citation13,Citation14 Economic analyses have generally shown that vaccination is cost-effective, particularly in the elderly.Citation15–Citation17
The US Centers for Disease Control and Prevention (CDC) recommend influenza vaccination for children aged between 6 months and 18 years, any adult who wants to reduce the risk of becoming ill with influenza or of transmitting it to others, adults aged 50 years and above, persons with conditions or receiving medication that put them at risk for influenza complications, residents of nursing homes and other chronic-care facilities, and contacts of these persons and healthcare workers.Citation18 However, uptake of vaccination continues to be low; although 73% of Americans were included in target vaccination groups in the 2006–2007 season, only about a third actually received vaccination.Citation6 In years where vaccine supply is limited, the CDC recommends that priority is given to persons at higher risk of influenza complications.Citation18 To achieve target vaccination rates, it is essential to ensure adequate vaccine supply. Some difficulties with influenza vaccine supply have occurred in recent years, highlighting the need to mitigate supply issues by the availability of vaccines from multiple manufacturers.
A trivalent inactivated split-virion influenza vaccine [Fluarix™, GlaxoSmithKline (GSK) Biologicals] has been available in Europe since 1992 (as Influsplit SSW® in Germany since 1987), and was registered in the US in 2005 under the accelerated approval regulation based on safety and immunogenicity demonstrated in a placebo-controlled study.Citation19 The newly-licensed vaccine is highly immunogenic in all populations studied and has a favorable safety profile.Citation20–Citation23 Under the accelerated approval regulation, more studies are required after registration to further demonstrate clinical benefit, including studies comparing the new vaccine with an established US-licensed vaccine. The present study was conducted to comply with this requirement, and compares the newly-licensed vaccine with Fluzone® (Sanofi Pasteur), a vaccine that is well established in the US.
Results
Demography.
A total of 923 subjects received the newly-licensed vaccine and 922 received the established vaccine, with 918 and 908, respectively, completing the study (). The first volunteer was enrolled on 14 October 2005, the last study visit took place on 29 November 2005, and the last safety follow-up data were obtained in June 2006. Demographic characteristics were similar between vaccine groups; median age was 68 years (range 18–96 years), 59% were women and 92% were white. A total of 635 subjects were aged 18–64 years (317 in the newly-licensed vaccine group and 318 in the established vaccine group) and 1,210 were aged ≥65 years (606 in the newly-licensed vaccine group and 604 in the established vaccine group).
The according to protocol (ATP) cohort for the immunogenicity analysis included 872 and 867 subjects in the newlylicensed vaccine and the established vaccine groups, respectively; reasons for exclusion from this cohort are shown in .
Immunogenicity.
Both vaccines induced a good immune response, and non-inferiority requirements were achieved for all three vaccine strains ( and ). In the analysis of the whole study population, pre-vaccination GMTs were similar in both vaccine groups for all three vaccine strains (H1N1: 27.9 and 29.1; H3N2: 16.3 and 16.5; B: 47.7 and 54.1 in the newlylicensed vaccine and the established vaccine groups, respectively) and rose substantially after vaccination. Post-vaccination GMTs and seroconversion rates were similar with both vaccines, although numerically higher with the newly-licensed vaccine with regard to H1N1 response. Post-vaccination GMTs with the newly-licensed vaccine were 138.0 (95% CI: 125.2, 152.1), 121.6 (110.5, 133.7) and 231.9 (215.4, 249.6) against the H1N1, H3N2 and B strains, respectively; corresponding values for the established vaccine were 92.0 (84.5, 100.3), 114.0 (104.4, 124.5) and 273.7 (253.4, 295.7) (). Seroconversion rates for the newly-licensed vaccine were 45.7% (42.3, 49.1), 67.1% (63.9, 70.3) and 52.7% (49.3, 56.1) against the H1N1, H3N2 and B strains, respectively; corresponding values for the established vaccine were 33.8% (30.6, 37.1), 65.5% (62.2, 68.7) and 53.8% (50.4, 57.2) ().
As the elderly tend to have lower immune responses compared with younger adults,Citation24 separate analyses of the GMT and seroconversion rates in the elderly population were performed to confirm the immunogenicity of the newly-licensed vaccine in these subjects. Although the vaccines were immunogenic in both study populations, lower immunogenicity was observed in subjects ≥65 years compared with the population as a whole with respect to both GMT and seroconversion rates ( and ). Again, though, among subjects aged ≥65 years, non-inferiority criteria for the newly-licensed vaccine compared with the established vaccine were achieved for all vaccine strains ().
We also found no substantial difference between the newly-licensed vaccine and the established vaccine with regard to sero-protection rates in the overall study population. Post-vaccination seroprotection rates (95% CI) with the newly-licensed vaccine and the established vaccine, respectively, were 87% (85, 89) and 82% (79, 84) for H1N1, 82% (79, 84) and 85% (82, 87) for H3N2, and 97% (96, 98) and 98% (96, 99) for B, for all subjects (≥18 years). The proportion of initially unprotected subjects (baseline hemagglutination inhibition (HI) titer <1:40) who achieved a minimum 4-fold increase in HI titer was 67% and 53% (H1N1), 77% and 76% (H3N2) and 80% and 84% (B) for the newly-licensed vaccine and the established vaccine, respectively, in the whole study population. This lack of difference between the two vaccines was further confirmed in the subanalyses of subjects grouped by their immunocompetency and prior vaccination status. Only 5.7% of the newly-licensed vaccine subjects and 6.7% of the established vaccine subjects were judged by investigators to be immunocompromised, and 82.5% and 84.6% respectively had been vaccinated against influenza in the past 3 years. In both immunocompromised and previously vaccinated subjects, post-vaccination GMTs increased significantly to a similar degree with both vaccines, although the immune response in these subject groups was reduced compared with immunocompetent or previously unvaccinated subjects (data not shown).
Analyses of non-inferiority performed on the total vaccinated cohort confirmed the results found in analyses performed on the ATP cohort (data not shown).
Safety.
Safety results are reported for the total vaccinated cohort. Complete diary card data for the 4-day post-vaccination period were available for 917 [for local adverse events (AEs)] and 916 (for general AEs) subjects receiving the newly-licensed vaccine and for 910 subjects receiving the established vaccine. Both vaccines were well tolerated and the majority of AEs were mild or moderate in intensity. Solicited and unsolicited AEs occurring during the 4-day post-vaccination period were reported by 48.5% of the newly-licensed vaccine subjects and 47.0% of the established vaccine subjects. Grade 3 AEs during this period were reported by only 1.4% and 2.9% of the newly-licensed vaccine and the established vaccine subjects, respectively. Solicited AEs are shown in . Pain was the local AE most frequently experienced, by approximately 29% of subjects in both vaccine groups. Slightly more of the subjects receiving the established vaccine reported swelling, grade 3 swelling and grade 3 redness compared with subjects receiving the newly-licensed vaccine; although the numerical differences were small, they were statistically significant (; grade 3 data not shown). All local AEs were considered to be related to study vaccine. Headache and fatigue were the most frequently reported general AEs (). Fever occurred at a slightly, but statistically significantly, higher rate in the newly-licensed vaccine group versus the established vaccine group, although the incidence was low in both groups (2.1% versus 0.7%) and no subjects experienced severe fever (>39°C).
Over the entire study period (up to 6 months), 132 serious adverse events (SAEs) occurred in 93 subjects (45 in the newly-licensed vaccine group and 48 in the established vaccine group), none of which were considered to be related to the study vaccines. Two deaths occurred during the extended follow-up period, both in the established vaccine group, but neither was considered to be related to the vaccine. Six subjects in the newly-licensed vaccine group and five in the established vaccine group reported new onset of chronic illness during the extended followup period, none of which were considered to be related to the study vaccines.
During the follow-up period up to the second study visit, 20.0% of the newly-licensed vaccine subjects and 19.6% of the established vaccine subjects reported unsolicited AEs (all AEs reported as starting after the initial 4-day follow-up period were designated “unsolicited”). Unsolicited AEs reported by >1% of subjects in either group were headache (2.8% and 2.3% of the newly-licensed vaccine and established vaccine subjects, respectively), nasopharyngitis (1.0% and 1.3%), pharyngolaryngeal pain (1.2% and 0.9%), back pain (1.5% and 0.4%), cough (1.1% and 0.9%), pain in an extremity (1.2% and 0.7%) and fatigue (1.1% and 0.7%). Adverse events judged to be related to study vaccine occurred in 3.6% and 4.2% of the newly-licensed vaccine and the established vaccine subjects, respectively, with the most common related AEs being injection site bruising and injection site pruritus.
Discussion
The study clearly showed that both vaccines were highly immunogenic. Geometric mean titers rose substantially following vaccination, and high rates of seroconversion and seroprotection were also achieved. The primary objective of this study, designed to satisfy post-marketing regulatory requirements of the US Food and Drug Administration (FDA) under its accelerated approval pathway, was to demonstrate non-inferiority of the newly-licensed vaccine Fluarix™ compared with Fluzone®, an established vaccine. This objective was achieved both in the overall study population and in a separate analysis of elderly subjects (≥65 years of age).
Seroconversion and seroprotection rates in the whole study population (≥18 years) with the newly-licensed vaccine in this study were consistent with those observed in European studies conducted over 10 yearsCitation22 and in the previous placebo-controlled study of the newly-licensed vaccine in the US.Citation19 In addition to demonstrating non-inferiority to an established vaccine, Center for Biological Evaluation and Research (CBER) licensing criteria require that the lower 95% CIs for seroconversion rate and seroprotection rate are ≥40% and ≥70%, respectively, for all three viral strains in adults <65 years of age. In the analysis of the whole population (≥18 years), these criteria were achieved with the newly-licensed vaccine for all three viral strains, although not with the established vaccine with regard to seroconversion against the H1N1 strain (point estimate 33.8%, 95% CI 30.6, 37.1). Reflecting this finding, there were some minor differences between the two vaccines in immunogenicity parameters. However, these differences were not considered to be clinically significant and did not affect the conclusion of non-inferiority.
It is well recognized that the elderly have a reduced immune response to a number of vaccines, including inactivated influenza vaccine,Citation12 tick-borne encephalitis vaccine,Citation24 hepatitis A/B vaccineCitation25 and diphtheria-pertussis-tetanus vaccine.Citation26 This is probably due, at least in part, to immunosenescence.Citation27 It was therefore not surprising that the immune response to both vaccines in this study was lower in elderly subjects than in the overall study population. For the population aged ≥65 years, CBER criteria require that the lower end of the 95% CI for seroconversion rate is ≥30%; this threshold was met by both vaccines for each of the three viral strains with the exception of the established vaccine for the H1N1 strain (point estimate 28.1%, 95% CI 24.4, 32.1). The age-related reduction in immune response in the present study is broadly in line with that reported elsewhere for the newly licensed vaccine.Citation23 Indeed, the seroconversion rates observed in elderly subjects were similar to those reported in the elderly in a quantitative review of antibody response to influenza vaccination in the elderly versus younger adults.Citation12
Both vaccines were well tolerated and most AEs were mild or moderate in intensity. Headache and fatigue were the most common general AEs, reported by 12–13% of subjects. Although there were some statistically significant differences between the newly-licensed vaccine and the established vaccine with regard to redness and swelling (in favor of the newly-licensed vaccine) and fever (in favor of the established vaccine), numerical differences were small and were not considered to be clinically relevant. The frequency of solicited AEs in this study was similar to that in the previous US trial of the newly-licensed vaccine.Citation19 Severe local symptoms were reported infrequently. Pain was the most common local AE, reported by 29% of subjects in both groups.
Studies of the established vaccine have shown that it provides protection against laboratory-confirmed influenza, including during seasons when there is a sub-optimal match between vaccine and circulating viral strains.Citation28,Citation29 Induction of HI antibodies is generally accepted as a surrogate endpoint for protection against influenza infection in the young adult population, although it has limitations as the sole indicator of influenza vaccine efficacy in the elderly.Citation30 As the immunogenicity of the established vaccine and the newly-licensed vaccine in this study were similar, it is reasonable to conclude that the newly-licensed vaccine will offer similar clinical protection against influenza as the established vaccine.
The newly-licensed vaccine is immunologically non-inferior and as well tolerated as an established vaccine that has been available for many years in the US. The newly-licensed vaccine achieved CBER criteria for seroconversion and seroprotection rates for all three vaccine strains in the whole study population (≥18 years) and in the elderly (≥65 years). With the completion of this study and a placebo-controlled study of the efficacy of the newly-licensed vaccine,Citation31 all US regulatory requirements for licensure of the new vaccine for use in adults have been completed.
Methods
We performed a study to evaluate the immunogenicity and safety of the newly-licensed vaccine compared with an established vaccine. The primary study objective was to demonstrate immunologic non-inferiority of the newly-licensed vaccine versus the established vaccine in all adults, and a secondary objective was to demonstrate immunologic non-inferiority in the elderly (≥65 years). Safety objectives were to compare rates of local and general reactogenicity and adverse events (AEs) among recipients of the two influenza vaccines.
The trial was conducted in accordance with International Conference on Harmonization Good Clinical Practice Guidelines. The protocol was approved by the Institutional Review Boards of the institutions taking part and all subjects provided written, informed consent.
Subjects.
Male and female volunteers aged ≥18 years were included in the study. Exclusion criteria of the study included receipt of any inactivated vaccine within the 2 weeks prior to study entry; receipt of any live vaccine within the 4 weeks prior to study entry; history of hypersensitivity to a previous influenza vaccine; history of allergic reaction to any component of the study vaccines; acute disease at enrollment; history of Guillain Barré syndrome within the 6 weeks following receipt of an inactivated influenza vaccine; or use of any investigational drug within 30 days before administration of study vaccine. Female subjects of childbearing potential had to be using adequate contraceptive methods, and pregnant or breast-feeding women were excluded from the study.
Study vaccines.
Both the newly-licensed vaccine (Fluarix™, GSK Biologicals) and the established vaccine (Fluzone®, Sanofi Pasteur) contained 15 µg of viral hemagglutinin (HA) for each of the virus strains recommended for the 2005–2006 Northern Hemisphere influenza season: A/New Caledonia/20/99 (H1N1), A/New York/55/2004 (H3N2), and B/Jiangsu/10/2003 (B). The newly-licensed vaccine was supplied in pre-filled syringes and the established vaccine in 5 mL multi-dose vials; participants received a single dose of one or the other vaccine (0.5 mL per dose) intramuscularly into the deltoid muscle of the non-dominant arm.
Study design.
This trial was a randomized, active-controlled, parallel-group, observer-blind study. Subjects were enrolled by 15 investigators at multiple sites throughout the US, and randomly assigned in a 1:1 ratio to receive the newly-licensed vaccine or the established vaccine using internet randomization coordinated by the sponsor (GSK Biologicals). The randomization algorithm used a minimization procedure that accounted for center, age (18–64 years and ≥65 years), prior vaccination status and immunocompetence, with each factor having equal weight in the algorithm.
Immunogenicity evaluation. Subjects visited the clinic on the day of vaccination (day 0) and again 21 to 28 days later. Blood samples were collected at both visits to evaluate the immune response. Antibodies against the three influenza virus strains contained in the vaccines were measured using the HI assay according to standardized methodsCitation32 at the central laboratory of GSK Biologicals (Dresden, Germany). For each vaccine strain, the following parameters were determined: (1) GMT with 95% CI of HI antibodies pre- and post-vaccination; (2) seroconversion rate with 95% CI, defined as the proportion of subjects with (a) pre- and post-vaccination HI titers <1:10 and ≥1:40, respectively or (b) a pre-vaccination titer ≥1:10 and a minimum 4-fold increase in post-vaccination titer; (3) seroprotection rate with 95% CI, defined as the proportion of subjects with a post-vaccination HI titer ≥1:40; (4) proportion of subjects whose baseline titer was <1:40 with a 4-fold increase in HI titer.
Safety outcomes. Subjects completed diary cards to record solicited and unsolicited adverse events (AEs) during a 4-day post-vaccination follow-up period. Local solicited AEs were pain, redness and swelling at the injection site. All injection site reactions were considered to be causally related to vaccination. Solicited general AEs were fatigue, fever, headache, myalgia, shivering and arthralgia. The maximum intensity of solicited AEs was scored on a scale of 0–3 (0 = absent, 1 = easily tolerated, 2 = interferes with normal activity, 3 = prevents normal activity). Redness and swelling were scored according to the size of the area affected (0 = 0 mm, 1 = >0−≤20 mm, 2 = >20−≤50 mm, 3 = >50 mm). The maximum intensity of fever was scored according to temperature (1 = ≥37.5−≤38.0°C, 2 = >38.0−≤39.0°C, 3 = >39.0°C). The intensity of other AEs was scored on a scale of 1–3 (1 = mild, 2 = moderate, 3 = severe). Any unsolicited AEs occurring from vaccination until the second visit were recorded. In addition, subjects were contacted 6 months after vaccination to inquire about the occurrence of new onset of chronic illness or serious adverse events (SAEs).
Statistical analysis.
Sample size calculations were based on a study conducted to support the annual registration of the newlylicensed vaccine in Europe (data not shown). Assuming that 10% of the subjects would be unevaluable, 820 subjects per vaccine group were needed to demonstrate the primary objective of immunologic non-inferiority (defined below) for the three vaccine strains. The sample size ensured a global type 1 error of less than 2.5% and a global power greater than 90%.
The primary immunogenicity analysis of non-inferiority of the newly-licensed vaccine versus the established vaccine was based on GMT ratio and seroconversion rates. Non-inferiority was defined in accordance with the US FDA's CBER criteria, which are applicable to all age groups: (1) the upper limit of the 2-sided 95% CI of the GMT ratio (established vaccine/newlylicensed vaccine) for all three vaccine strains should not exceed 1.5; (2) the upper limit of the 2-sided 95% CI for the difference in seroconversion rates (established vaccine minus newly-licensed vaccine) for all three vaccine strains should not exceed 10%. Non-inferiority of the newly-licensed vaccine in the elderly (≥65 years) was determined in the same way (secondary analysis). The primary immunogenicity analysis was based on the according to protocol (ATP) cohort, which included all evaluable subjects for whom post-vaccination assay results for antibodies against at least one vaccine strain were available. Evaluable subjects included those who met all eligibility criteria, complied with study procedures, had not received any investigational drug or vaccines in accordance with the exclusion criteria, and had not received immunoglobulin or blood products during the study period up to the second visit.
The primary safety analysis was based on the total vaccinated cohort, which included all subjects for whom safety data were available. A 2-sided Fisher's exact p-value or 2-sided asymptotic p-value and the standardized asymptotic 95% CIs for the difference between vaccine groups were calculated for solicited AEs during the 4 day follow-up period. Exact 95% CIs were calculated for unsolicited AEs occurring during the follow-up period up to the second study visit.
The exact 95% CIs for proportions within a group and the standardized asymptotic 95% CIs for group differences in proportions were calculated using Proc StatXact 5.0. The 95% CI for the GMT for each group was obtained by exponential transformation of the 95% CI for the mean of the log-transformed titer. The group GMT ratio was obtained using an ANCOVA model on the log-transformed titers, and the 95% CI for the adjusted GMT ratio was obtained by exponential-transformation of the 95% CI for the group least-squares mean of the ANCOVA model. The ANCOVA model allowed adjustment for baseline titer and included the vaccine group as a fixed effect and the prevaccination log-transformed titer as regressor.
Competing interests
J.D.C. has provided consultative services for GSK with respect to vaccines and other products and his institution has received payment. C.V.C., M.C.C. and N.L.B. have provided consultative services for GSK and their institutions have received grant support from GSK to perform this and other vaccine studies. R.C.B. has received grant support from GSK to perform this and other vaccine studies. M.F., V.K.J. and B.L.I. are employees of GSK Biologicals. B.L.I. and V.K.J. report ownership of stock options.
Authors' Contributions
J.D.C.: Performance of study, collection and review of data, creation of tables and figures, writing of abstract and presentation of data at scientific meeting
C.V.C.: Performance of study, collection and review of data
R.C.B.: Performance of study, collection and review of data
M.C.C.: Performance of study, collection of data, data analysis, creation and review of tables and figures, writing of abstract and review of data for presentation at scientific meeting
N.L.B.: Collection and review of data
M.F.: Input into study design and protocol, validation of reporting and analysis plan, statistical report and clinical report
V.K.J.: Study design, medical monitor for the sponsor, data analysis
B.L.I.: Study design, data analysis.
All authors reviewed and contributed to a draft of the manuscript, and read and approved the final manuscript.
Trial Registration
NCT00197288.
Abbreviations
| AEs | = | Adverse Events |
| ATP | = | According to Protocol |
| B | = | B/jiangsu/10/2003 |
| CBER | = | Center for Biological Evaluation and Research |
| CDC | = | Centers for Disease Control and Prevention |
| CI | = | Confidence Interval |
| FDA | = | Food and Drug Administration |
| GMT | = | Geometric Mean Titer |
| GSK | = | GlaxoSmithKline |
| H1N1 | = | A/new caledonia/20/99 |
| H3N2 | = | A/new york/55/2004 |
| HA | = | Hemagglutinin |
| HI | = | Hemagglutination Inhibition |
| SAEs | = | Serious Adverse Events |
| US | = | United States |
Figures and Tables
Figure 1 Disposition of subjects. ATP: according to protocol; AE: adverse event; SAE: serious adverse event.
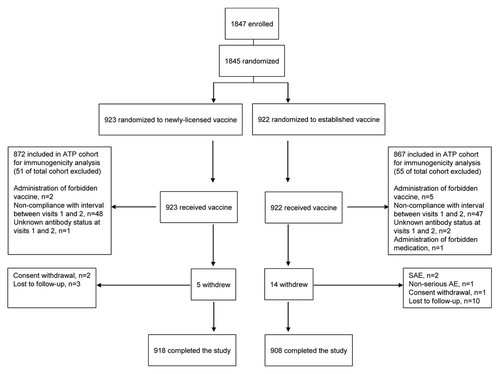
Figure 2 Non-inferiority analysis of the newly-licensed vaccine versus the established vaccine for adjusted geometric mean titer ratio. All subjects (≥18 years), according to protocol cohort. H1N1: A/New Caledonia/20/99; H3N2: A/New York/55/2004; B: B/Jiangsu/10/2003. Adjusted GMT: geometric mean antibody titer adjusted for baseline titer; 95% CI: 95% confidence interval; GMT ratio: established vaccine/newlylicensed vaccine Solid square indicates the point estimate of the GMT and hash marks the limits of the 95% confidence intervals. Dashed line indicates non-inferiority limit. Non-inferiority was defined as: the upper limit of the 2-sided 95% CI of the GMT ratio (established vaccine/newly-licensed vaccine) for all three vaccine strains should not exceed 1.5.
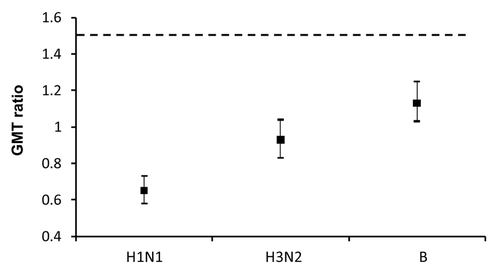
Figure 3 Geometric mean titers measured at the second study visit (days 21–28). Data presented separately for all subjects (≥18 years) and for the elderly (≥65 years), according to protocol cohort. Values were calculated in subjects who had a serum sample available at the second study visit: Newly-licensed vaccine, all subjects (≥18 years), n = 866. Established vaccine, all subjects (≥18 years), n = 854. Newly-licensed vaccine, elderly subjects (≥65 years), n = 567. Established vaccine, elderly subjects (≥65 years), n = 550. H1N1: A/New Caledonia/20/99; H3N2: A/New York/55/2004; B: B/Jiangsu/10/2003. Error bars: 95% confidence interval.
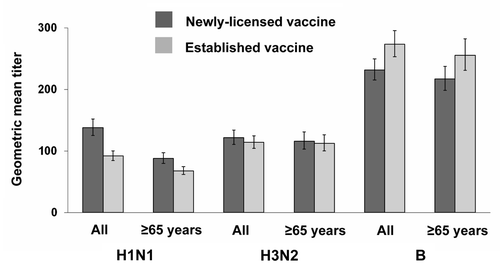
Figure 4 Seroconversion rates measured at the second study visit (days 21–28). Data presented separately for all subjects (≥18 years) and for the elderly (≥65 years), according to protocol cohort. Values were calculated in subjects who had serum samples available at baseline and at the second study visit. Newly-licensed vaccine, all subjects (≥18 years), n = 858. Established vaccine, all subjects (≥18 years), n = 846. Newly-licensed vaccine, elderly subjects (≥65 years), n = 562. Established vaccine, elderly subjects (≥65 years), n = 544. H1N1: A/New Caledonia/20/99; H3N2: A/New York/55/2004; B: B/Jiangsu/10/2003. Seroconversion: 4-fold rise in titer and ≥1:40. Error bars: 95% confidence interval.
Table 1 Non-inferiority analysis for adjusted geometric mean titer ratio and difference in seroconversion rate
Table 2 Percentage of subjects reporting a solicited adverse event during the 4 day post-vaccination period
Acknowledgements
The authors gratefully acknowledge all the study participants. We are also indebted to the participating clinicians, nurses and laboratory technicians at the study sites and the sponsor's project staff for their support and contributions throughout the study. We also want to thank Mary Greenacre (An Sgriobhadair Ltd.,) who provided medical writing services on behalf of GlaxoSmithKline (GSK) Biologicals, and Isabelle Camby, Luise Kalbe and Isabelle Gautherot (GSK Biologicals) for coordination of manuscript development.
GSK Biologicals was the funding source of this study and was involved in all stages of its conduct and analysis. GSK Biologicals also took responsibility for all costs associated with the development and publishing of the present manuscript. All authors had full access to the data and had the final responsibility to submit for publication.
Fluarix is a trademark of the GlaxoSmithKline group of companies. Fluzone is a registered trademark of Sanofi Pasteur Inc.
References
- World Health Organization. WHO 2003; [http://www.who.int/mediacentre/factsheets/fs211/en]. Fact sheet no 211
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 186
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333 - 1340
- Mullooly JP, Bridges CB, Thompson WW, Chen J, Weintraub E, Jackson LA, et al. Influenza- and RSV-associated hospitalizations among adults. Vaccine 2007; 25:846 - 855
- O'Brien MA, Uyeki TM, Shay DK, Thompson WW, Kleinman K, McAdam A, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113:585 - 593
- Centers for Disease Control and Prevention: Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) 2007. MMWR 2007; 56:1 - 54
- Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA 2000; 284:1655 - 1663
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2007; 1269
- Beran J, Wertzova V, Honegr K, Kaliskova E, Havlickova M, Havlik J, et al. Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis 2009; 9:2
- Skowronski DM, Masaro C, Kwindt TL, Mak A, Petric M, Li Y, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine 2007; 25:2842 - 2851
- Herrera GA, Iwane MK, Cortese M, Brown C, Gershman K, Shupe A, et al. Influenza vaccine effectiveness among 50–64 year-old persons during a season of poor antigenic match between vaccine and circulating influenza virus strains: Colorado, United States 2003–2004. Vaccine 2007; 25:154 - 160
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159 - 1169
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the communitydwelling elderly. N Engl J Med 2007; 357:1373 - 1381
- Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine 2002; 20:1831 - 1836
- Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine 2003; 21:1769 - 1775
- Nichol KL, Nordin J, Mullooly J. Influence of clinical outcome and outcome period definitions on estimates of absolute clinical and economic benefits of influenza vaccination in community dwelling elderly persons. Vaccine 2006; 24:1562 - 1568
- Rothberg MB, Rose DN. Vaccination versus treatment of influenza in working adults: a cost-effectiveness analysis. Am J Med 2005; 118:68 - 77
- Centers for Disease Control and Prevention: Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) 2008. MMRW 2008; 57:1 - 60
- Treanor JJ, Campbell JD, Brady RC, Keitel WA, Drame M, Jain VK, et al. Rapid licensure of a new, inactivated influenza vaccine in the United States. Hum Vaccin 2005; 1:239 - 244
- Baras B, Bouveret N, Devaster JM, Fries L, Gillard P, Sänger R, et al. A vaccine manufacturer's approach to address medical needs related to seasonal and pandemic influenza viruses. Influenza Other Respi Viruses 2008; 2:251 - 260
- El Sahly HM, Keitel WA. Clinical data on Fluarix: an inactivated split seasonal influenza vaccine. Expert Rev Vaccines 2008; 7:713 - 719
- Hehme NW, Künzel W, Petschke F, Turk G, Raderecht C, Van Hoecke C, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Invest 2002; 22:751 - 769
- Rose GW, Cooper CL. Fluarix, inactivated split-virus influenza vaccine. Expert Opin Biol Ther 2006; 6:301 - 310
- Weinberger B, Keller M, Fischer KH, Stiasny K, Neuner C, Heinz FX. Decreased antibody titers and booster responses in tick-borne encephalitis vaccinees aged 50–90 years. Vaccine 2010; 28:3511 - 3515
- Wolters B, Junge U, Dziuba S, Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine 2003; 21:3623 - 3628
- Kaml M, Weiskirchner I, Keller M, Luft T, Hoster E, Hasford J, et al. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine 2006; 24:6808 - 6811
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46:1078 - 1084
- Belongia E, Kieke B, Coleman L, Donahue J, Irving S, Meece J, et al. Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine—Marshfield, Wisconsin 2007–08 influenza season. MMWR 2008; 57:393 - 397
- Strickler JK, Hawksworth AW, Myers C, Irvine M, Ryan MA, Russell KL. Influenza vaccine effectiveness among US military basic trainees 2005–06 season. Emerg Infect Dis 2007; 13:617 - 619
- McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take?. J Infect Dis 2008; 198:632 - 634
- Beran J, Vesikari T, Wertzova V, Karvonen A, Honegr K, Lindblad N, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective randomized placebo-controlled trial. J Infect Dis 2009; 200:1861 - 1869
- Kendal AP, Pereira MS, Skehel JJ. Concepts and procedures for laboratory-based influenza surveillance 1982; Atlanta Centers for Disease Control and Prevention and Pan-American Health Organization 17 - 35