Abstract
The anti-tumor function of STAT1 through its capacity to control the immune system and promote tumor immune surveillance has been well understood. However, little is known about cell autonomous (i.e., tumor cell-specific) functions of STAT1 in tumor formation. Recent studies have provided strong evidence that STAT1 suppresses mouse mammary gland tumorigenesis by both, immune regulatory and tumor cell-specific functions of STAT1. Specifically, STAT1 deficiency in the mouse mammary gland inhibits ErbB2/Neu-mediated tumorigenesis and contributes to spontaneous formation of estrogen receptor α (ER α)-positive as well as ER α-negative tumors closely resembling human disease. Herein, we review the anti-tumor functions of STAT1 revealed from investigations of murine breast cancer models and from characterization of the signaling properties of STAT1 in human breast tumor cells. The significance of STAT1 in breast cancer is underscored by studies proposing a prognostic value for the expression and/or phosphorylation of STAT1 for specific molecular types of breast cancer. Furthermore, STAT1 dependent transcription is proposed to contribute to therapeutic responses by modulating the efficacy of chemotherapeutic drugs and the development of drug resistance.
The Anti-Tumor Properties of STAT1
The signal transducers and activators of transcription (STATs) belong to a family of seven cytoplasmic proteins that function as signal messengers and transcription factors participating in cellular responses to cytokines and growth factors.Citation1,Citation2 The prototypical member STAT1 plays an essential role in innate immunity by protecting the host from virus infections and other pathogens.Citation1,Citation2 Via DNA-binding STAT1 acts downstream of type I and II interferon (IFN) receptors and mediates the transcription of genes, which encode proteins with anti-proliferative, anti-viral and immune regulatory properties.Citation1,Citation2 STAT1 activity is controlled by phosphorylation at tyrosine (Y) 701 and serine (S) 727 within the transactivation domain (TAD) of the protein.Citation1,Citation2 Phosphorylation determines the transition of STAT1 between different dimer conformations. Whereas unphosphorylated STAT1 may dimerize in an antiparallel conformation Y701 phosphorylation triggers a parallel dimer conformation mediated by the phosphotyrosine:SH2 domain interactions, allowing increased DNA binding and nuclear retention of the protein.Citation3,Citation4 Tyrosine phosphorylated STAT1 can hetero-dimerize by virtue of a reciprocal SH2:phosphotyrosine interaction with other STAT family members such as STAT2 and STAT3 to control cytokine and growth factor signaling.Citation2,Citation5 STAT1 Y701 phosphorylation is mediated by the receptor associated Janus kinases (JAKs) that are activated in response to IFNs and other cytokines.Citation1,Citation2 However, receptors with intrinsic tyrosine kinase activity (RTKs), such as epidermal growth factor receptor (EGFR), can also mediate STAT1 phosphorylation at Y701.Citation1,Citation2 STAT1 phosphorylation at S727 plays an essential role in gene transactivation in response to IFNs.Citation1,Citation2 STAT1 acetylation at lysine (K) 410 and K413 negatively regulates its activity by impeding DNA binding and promoting an anti-parallel conformation of STAT1 dimers that facilitates Y701 dephosphorylation.Citation6
Early studies revealed an increased susceptibility of STAT1−/− mice to chemical carcinogenesis compared with their wild type (WT) counterparts.Citation7 The increased rate of tumor formation in STAT1−/− mice was attributed to impaired immune surveillance of tumors because these mice fail to respond to IFN-γ and display reduced natural-killer (NK) activity.Citation8 Mating of STAT1−/− and p53−/− mice yields animals with an increased tumor incidence and a broader spectrum of tumors than p53−/− mice.Citation7 At the molecular level, STAT1 inhibits the proliferation of both mouse and human tumor cells treated with IFN-γ via its ability to increase the expression of cyclin dependent kinase (Cdk) inhibitor p21Cip1 Citation9 or to decrease c-myc expression.Citation10 Also, STAT1 promotes apoptosis by upregulating the expression of caspases 2,3 and 7Citation11,Citation12 or the expression of inducible nitric oxide synthase (iNOS).Citation13 The anti-tumor activity of STAT1 is further supported by its ability to inhibit angiogenesis and tumor metastasis in mouse models.Citation14 Early findings proposed a link between STAT1 phosphorylation and tumor suppression based on the fact that STAT1 was found to be phosphorylated at Y701 in various types of blood and solid human tumors.Citation15,Citation16 Although so far STAT1 mutants have not been described in human cancer, regulation of STAT1 activity by phosphorylation at Y701 and S727 plays a key role in Ras tumor formation.Citation17-Citation20 Despite the fact that the majority of studies support an anti-tumor function, STAT1 has been shown to promote leukemogenesis in mice expressing v-Abl or TEL-JAK2 oncoprotein.Citation20 This unusual property of STAT1 was found to be immune-dependent and require natural killer (NK) cell function and tumor immunoediting via the regulation of the major histocompatibility complex (MHC) class 1 expression independent of IFN signaling.Citation20
STAT1 and Breast Cancer
Studies on mouse mammary gland revealed that while STAT1 expression is maintained through pregnancy, lactation and involution, Y701 phosphorylation and DNA binding are only detected in virgin animals, or during early pregnancy and late involution.Citation21 Although STAT1 is regulated during the different stages of breast development,Citation21 STAT1−/− mice have a regular mammary gland development. In contrast to normal untransformed mammary cells STAT1 has been implicated in breast cancer development based on the observation that STAT1 Y701 phosphorylation is elevated in human breast tumorsCitation15 and is associated with an increased survival independent of other known prognostic factors.Citation22 Also, increased STAT1 mRNA levels were shown to be part of a molecular signature associated with better prediction of the metastatic outcome for patients with hormone receptor negative and triple-negative breast cancers.Citation23
Cell autonomous function of STAT1 in breast tumorigenesis
Recent studies performed independently in the laboratories of Hennighausen,Citation24 Koromilas,Citation25 SchreiberCitation26 and SexlCitation27 shed light on the role of STAT1 in mammary tumor formation. Despite the use of different experimental approaches, all studies reached similar conclusions and confirmed the tumor suppressing role of STAT1 in mammary tumorigenesis. Hennighausen and colleagues first reported the generation of mice bearing a floxed (flx) allele of STAT1.Citation24 The STAT1flx/flx mouse was crossed to a mouse expressing the ErbB2/neu oncogene (deemed NIC) under the control of the mouse mammary tumor virus (MMTV) promoter.Citation24 The MMTV-NIC mouse was originally designed to express Cre recombinase under the control of an internal ribosome entry site (IRES) from the same di-cistronic mRNA with the NIC oncogene (MMTV-NIC-IRES-Cre).Citation28 Mating of STAT1flx/flx mice with MMTV-NIC-IRES-Cre mice on FVB background resulted in STAT1 deletion within the same mammary epithelial cell expressing NIC.Citation24 Although tumors were first detected in both groups 36 weeks after birth, the overall disease latency was significantly enhanced in STAT1-deficient mice being 49.4 weeks compared with 62.4 weeks in STAT1-proficient animals. Since all cells of the tumor microenvironment expressed STAT1 with this approach, the anti-tumor role of STAT1 was thereby unequivocally linked to its cell intrinsic properties within the mammary epithelium.
Similarly, the Koromilas group used an in vivo model of tumorigenesis in which transgenic mice expressing an active form of ErbB2/Neu (deemed Neu NDL2-5) under the control of the MMTV promoterCitation29 were crossed with STAT1+/+ and STAT1−/− mice on Balb/c background.Citation30 They observed that Neu NDL2-5-positive STAT1−/− females that had borne one litter of pups developed tumors ~6 weeks before their STAT1+/+ counterparts (27 vs. 33 weeks).Citation25 The decreased latency of tumor formation was amplified in virgin females, with STAT1−/− mice developing tumors at a mean time of 41.6 weeks compared with 49.1 weeks for the STAT1+/+ mice. Once tumor development began, there was no appreciable difference in the number of tumors formed or the size of each tumor.Citation25 These results showed that STAT1 acts as a suppressor of Neu NDL2-5 mammary gland tumor formation in mice.Citation25 Increased tumor formation in STAT1−/− mice was not caused by morphological alterations of the mammary gland in the animals consistent with previous observations that STAT1−/− mice have no defects in the mammary gland development.Citation21
The Schreiber and Sexl groups analyzed spontaneous mammary tumor development to evaluate the impact of STAT1 in vivo.Citation26,Citation27 The Schreiber group reported a higher tumor incidence more than 90% in the parous animals compared with 55% of tumor incidence reported by the Sexl group. While Sexl and colleagues never observed a tumor in a virgin female Schreiber and colleagues also reported on a tumor incidence of 65% in virgin animals. Slight differences were also found in the receptor expression of the tumors in both studies; whereas the tumors in the Schreiber study displayed an estrogen receptor positive (ER)+ phenotype and closely resembled human progesterone receptor (PR)+/ER+ tumors, the STAT1−/− tumors described by the Sexl group were only in 50% ER+. The cause of these differences is not presently known but may be related to the different genetic background of the animals, that is, 129S6/SvEV background for the Schreiber studies vs. Balb/c background for the Sexl studies.
Both studies by the Schreiber and Sexl labs reported an enhanced appearance of mammary intraepithelial neoplasias (MINs) from the loss of STAT1 ().Citation26,Citation27 MINs represent precancerous lesions that have the potential to develop into carcinomas and are thought to result from increased proliferation and/or decreased apoptosis of the mammary epithelial cells. STAT1 has been implicated in both processes through its capacity to induce the expression of genes that inhibit cell proliferation and/or induce apoptosis.Citation13 A transcriptional role of STAT1 in mammary gland apoptosis was implied by the findings of the Schreiber group in which the transcriptionally inactive mutant of STAT1 (Y701F) was incapable of apoptosis induction.Citation26 However, the Sexl group did not detect any signs of cell death or apoptosis in their experimental system. In contrast, they were able to observe enhanced proliferation in vivo in both non-tumorigenic and tumorigenic mammary epithelial STAT1−/− cells.Citation27 Further confirmation for a role of STAT1 as key regulator of mammary epithelial cell proliferation came from three-dimensional (3D) culture studies. The Sexl group used the 3D technique to compare the formation of mammospheres from primary mammary epithelial cells of virgin STAT1−/− vs. STAT1+/+ mice. They found that STAT1−/− cells formed significantly thicker mammosphere layers with an increased proliferative rate and in some cases capable of fill in the lumen of the acini.Citation27 The 3D culture approach may prove useful for studying the mechanisms of MIN formation of STAT1 deficient epithelial cells.
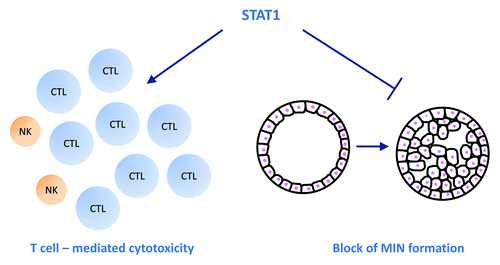
Figure 1. STAT1 counteracts spontaneous mammary tumor formation in a dual way. First, STAT1 is needed to sustain efficient cytotoxicity of CD8+ T cell which are the main executors of mammary tumor surveillance. Natural killer (NK) cells only play a marginal role in defending mammary tumorigenesis. Second, STAT1 counteracts mammary intraepithelial neoplasia (MIN) formation by regulating growth control of mammary epithelial cells and by inducing apoptosis in malignant cells.
Stroma effects of STAT1 in breast tumorigenesis
Considering that the anti-tumor activity of STAT1 is linked to its ability to promote tumor immune surveillance,Citation8 Koromilas and colleagues further examined the contribution of STAT1 in the stromal compartment in the inhibition of Neu NDL2-5-mediated mammary tumorigenesis. To this end, mammary tumor cells isolated from Neu NDL2-5 STAT1+/+ and STAT1−/− mice were subjected to orthotopic transplantation assays in syngeneic STAT1+/+ or STAT1−/− mice. They observed that growth of the transplanted tumors did not significantly differ between STAT1+/+ and STAT1−/− tumor cells in STAT1+/+ recipient mice kept under observation for ~9 weeks.Citation25 However, tumor growth of transplanted Neu NDL2-5 STAT1−/− mammary tumor cells under the same period of time was significantly induced compared with STAT1+/+ mammary tumor cells in STAT1−/− recipient mice.Citation25 Histopathology analysis of breast tumors identified two tumor phenotypes, one typical solid, nodular ErbB2/Neu-type tumor and another glandular papillary tumor, which were not different between STAT1+/+ and STAT1−/− mice.Citation25 These data revealed the presence of both stromal and tumor-site specific effects in the suppression of Neu NDL2-5-mediated mammary tumorigenesis by STAT1.
Further evidence for a key role of STAT1 in the tumor microenvironment was obtained from the studies of the Schreiber and Sexl groups.Citation26,Citation27 The Schreiber group reported on the selective downregulation of STAT1 protein in a large cohort of patients using histochemistry in tumor biopsies.Citation26 STAT1 downregulation was most prominent in the tumor cells themselves when compared with the surrounding stroma and infiltrating lymphocytes. The expression of STAT1 in the tumor stroma and the infiltrating lymphocytes may explain why many researchers have found high STAT1 levels in breast cancer samples under experimental conditions that do not allow separation of tumor stroma cells from the tumor cells such as western blotting or micro arrays of total tumors. These data highlighted the key role of the microenvironment for tumor development and the need of performing single cell resolution analysis in order to determine protein expression to a distinct cellular compartment. The Sexl group showed that STAT1-deficiency suffices to significantly enhance the incidence of spontaneous mammary tumor development in mice.Citation27 Moreover, the same group revealed a dual function of STAT1 in suppressing mammary tumor formation: on one hand STAT1 sustained efficient cytotoxic T lymphocyte (CTL)-dependent tumor surveillance and was required for full-fledged T cell cytotoxicity and on the other hand STAT1 inhibited cell proliferation in the mammary epithelial cells in a cell autonomous manner.Citation27 The ability of STAT1 to control the function of immune cells may play important role in regulation of tumorigenesis. That is, sustained activation of CTL by STAT1 contributes to breast tumor suppression. In contrast STAT1 loss in leukemic cells provokes the strong activation of natural killer (NK) cells due to the downreguation of MHC 1 molecules.Citation20
Mechanisms of tumor suppression by STAT1
To understand the anti-tumor mechanism of STAT1, Koromilas and colleagues addressed the regulation of STAT1 phosphorylation in ErbB2-mediated signaling. They found that STAT1 Y701 phosphorylation is decreased in ErbB2-positive human breast tumor cells after treatment with drug inhibitors of ErbB2 but not after treatments with inhibitors of Src or JAKs.Citation25 These data indicated that ErbB2 activation mediates signaling pathways leading to increased STAT1 Y701 phosphorylation, a notion that was further supported in transient expression assays of STAT1 and an activated form of Neu.Citation25 Although the same authors provided evidence that STAT1 is a substrate of EGFR in vitro, it remains unclear whether increased STAT1 Y701 phosphorylation in breast tumor cells is a direct effect of ErbB2.Citation25 The functional interactions between ErbB2 and STAT1 were further supported by recent findings showing that STAT1 expression is induced by ErbB2 at the transcriptional level through STAT3 activation.Citation31
In an attempt to explain STAT1 function downstream of ErbB2/Neu, the Koromilas group examined the effects of phosphorylation defective mutants of STAT1, namely STAT1Y701F and STAT1S727A, in ErbB2/Neu-mediated transformation. They found that the transforming properties of Neu NDL2-5 in p53−/−STAT1−/− mouse embryonic fibroblasts (MEFs) were efficiently impaired by the expression of the STAT1 phosphorylation mutants in xenograft tumor assays in immune compromised SCID mice.Citation25 This finding suggested that STAT1 can inhibit ErbB2/Neu-mediated tumorigenesis independent of phosphorylation at either Y701 or S727. Because the signaling networks of ErbB2/Neu and STAT1 are context-dependent, it remains possible that the function of phosphorylated STAT1 in MEFs is different from its function in mammary epithelial cells. The ErbB2/Neu-mediated transformation studies were performed in p53-deficient MEFs.Citation25 Therefore, it would be important to examine the effects of STAT1 phosphorylation mutants in Neu NDL2-5-transformed STAT1−/− mammary epithelial cells with wild-type p53.Citation32 An intriguing observation made by the Koromilas group was the ability of STAT1 to be phosphorylated by Neu NDL2-5 at tyrosine residues other than Y701 in transiently transfected cells.Citation25 To date, there has been little information about the regulation of STAT1 by phosphorylation at sites other than Y701 and S727. Previous studies by the Maniatis group showed the phosphorylation of STAT1 by IKKε at S708, a modification that contributes to the transcriptional induction of a specific set of IFN-inducible genes.Citation33 It remains possible that ErbB2 signaling leads to phosphorylation of STAT1 at novel sites that contribute to its anti-tumor activity in the mammary gland.
The Sexl group proposed that the inhibitory effects of STAT1 on ER-positive mammary epithelial cell growth are mediated by the interferon regulatory factor 1 (IRF-1).Citation27 Given that IRF-1 is induced by STAT1 at the transcriptional level, this explanation is consistent with the findings of the Schreiber group that inhibition of ER+ mouse tumor growth requires a transcriptionally active STAT1.Citation26 A role of IRF1 was shown experimentally by the significant downregulation of IRF-1 in STAT1-deficient tumors and the structural similarities between STAT1−/− and IRF1−/− mammary tissues in the 3D mammospheres.Citation27 A potential role of IRF1 in breast cancer was further supported by observations that IRF1 is frequently heterozygous in human breast tumor tissue and its expression is downregulated in high grade breast cancers.Citation27 Confirmation of the IRF1 function downstream of STAT1 would require the use of STAT1−/−IRF1−/− mice to examine the effect of deficiency of the STAT1-IRF1 axis on mammary tumor growth. Also, it would be of interest to determine whether IRF1 downregulation in human breast tumors is associated with decreased STAT1 expression or expression of a transcriptionally inactive STAT1.
Conclusions and Perspectives
In summary the evidence that STAT1 is a bona fide tumor suppressor of breast tumorigenesis is consistent and convincing. All studies examining mouse mammary tumorigenesis demonstrated the crucial role of STAT1 in the tumor initiating phase. This conclusion is further supported by recent findings showing that the anti-tumor activity of the milk protein α-casein requires the function of STAT1 in breast tumor cells.Citation34 It will be interesting to determine if and how STAT1 influences the progression of an already established breast cancer. Tumor cells frequently display alterations that help them to escape immune control such as the downregulation of IFN receptors that has been described in many tumor types. IFNs are considered major players in tumor surveillance with STAT1 being a key transcription factor mediating IFN-effects. Nevertheless, the effects of STAT1 on mammary tumor development do not simply reflect the inability to react to IFNs and are beyond the control of IFNs.
STAT1 responds to treatments with genotoxic drugsCitation35-Citation37 including doxorubicin,Citation38 a drug that is efficient for the treatment of ErbB2-positive human breast tumors.Citation39-Citation41 Therefore, it will be important to determine how STAT1 affects therapies with chemotherapeutic drugs used for the treatment of breast cancer. This will be possible with the treatment of the existing mouse models of breast cancer in which STAT1 is either systemically or conditionally deleted in the mammary gland. In addition, it would be of interest to examine whether STAT1 phosphorylation status determines the outcome of anti-breast tumor therapies with genotoxic drugs or drugs targeting ErbB2 and other receptor tyrosine kinases implicated in breast tumorigenesis.
An ability of STAT1 to sensitize breast tumor cells to chemotherapies aimed at the destruction of ER positive or ErbB2-positive tumors may be of therapeutic value by increasing the sensitivity of treatments and decreasing side effects. Also, further characterization of the known phosphorylation sites as well as identification and characterization of novel phosphorylation sites of STAT1 in ErbB2-positive breast cancers may be of diagnostic value for disease progression and treatment. This notion is supported by a recent study providing evidence that the cytoplasmic localization of Y701 phosphorylated STAT1 is a prognostic factor of advanced stage and worse survival of premenopausal breast cancer patients.Citation42 Moreover, identification of genes that mediate the suppressor properties of STAT1 in breast tumor cells may prove of prognostic value for the implementation of therapies that maximize the activation capacity of STAT1. Given that expression of a specific set of IFN-inducible genes by STAT1 has been implicated in drug resistanceCitation35-Citation37 and decreased metastasis-free survival of breast cancer patients,Citation43 transcriptional profiling of STAT1 may provide useful information about the implementation of efficacious therapies to combat breast cancer.
Acknowledgments
The work was supported by a grant from the Canadian Breast Cancer Research Alliance (CBCRA) administered via the Canadian Institutes of Health Research (CIHR), a grant from Quebec Breast Cancer Foundation (QBCF) and a pilot study grant from the Weekend to End Women’s Cancer (WEWC) Research Fund of the Segal Cancer Center to A.E.K. and by project SFB-28-10 by the Austrian Science Fond (FWF) to V.S.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem 2007; 282:20059 - 63; http://dx.doi.org/10.1074/jbc.R700016200; PMID: 17502367
- Stark GR, Darnell JE Jr.. The JAK-STAT pathway at twenty. Immunity 2012; 36:503 - 14; http://dx.doi.org/10.1016/j.immuni.2012.03.013; PMID: 22520844
- Wenta N, Strauss H, Meyer S, Vinkemeier U. Tyrosine phosphorylation regulates the partitioning of STAT1 between different dimer conformations. Proc Natl Acad Sci U S A 2008; 105:9238 - 43; http://dx.doi.org/10.1073/pnas.0802130105; PMID: 18591661
- Zhong M, Henriksen MA, Takeuchi K, Schaefer O, Liu B, ten Hoeve J, et al. Implications of an antiparallel dimeric structure of nonphosphorylated STAT1 for the activation-inactivation cycle. Proc Natl Acad Sci U S A 2005; 102:3966 - 71; http://dx.doi.org/10.1073/pnas.0501063102; PMID: 15753310
- Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol 2008; 19:351 - 9; http://dx.doi.org/10.1016/j.semcdb.2008.06.004; PMID: 18620071
- Krämer OH, Heinzel T. Phosphorylation-acetylation switch in the regulation of STAT1 signaling. Mol Cell Endocrinol 2010; 315:40 - 8; http://dx.doi.org/10.1016/j.mce.2009.10.007; PMID: 19879327
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 1998; 95:7556 - 61; http://dx.doi.org/10.1073/pnas.95.13.7556; PMID: 9636188
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6:836 - 48; http://dx.doi.org/10.1038/nri1961; PMID: 17063185
- Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 1996; 272:719 - 22; http://dx.doi.org/10.1126/science.272.5262.719; PMID: 8614832
- Ramana CV, Grammatikakis N, Chernov M, Nguyen H, Goh KC, Williams BR, et al. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J 2000; 19:263 - 72; http://dx.doi.org/10.1093/emboj/19.2.263; PMID: 10637230
- Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol 1997; 17:5328 - 37; PMID: 9271410
- Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 1997; 278:1630 - 2; http://dx.doi.org/10.1126/science.278.5343.1630; PMID: 9374464
- Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cell Signal 2007; 19:454 - 65; http://dx.doi.org/10.1016/j.cellsig.2006.09.003; PMID: 17085014
- Huang S, Bucana CD, Van Arsdall M, Fidler IJ. Stat1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene 2002; 21:2504 - 12; http://dx.doi.org/10.1038/sj.onc.1205341; PMID: 11971185
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer 2004; 4:97 - 105; http://dx.doi.org/10.1038/nrc1275; PMID: 14964307
- Bromberg J. Stat proteins and oncogenesis. J Clin Invest 2002; 109:1139 - 42; PMID: 11994401
- Wang S, Koromilas AE. Stat1 is an inhibitor of Ras-MAPK signaling and Rho small GTPase expression with implications in the transcriptional signature of Ras transformed cells. Cell Cycle 2009; 8:2070 - 9; http://dx.doi.org/10.4161/cc.8.13.8891; PMID: 19502801
- Wang S, Raven JF, Durbin JE, Koromilas AE. Stat1 phosphorylation determines Ras oncogenicity by regulating p27 kip1. PLoS One 2008; 3:e3476; http://dx.doi.org/10.1371/journal.pone.0003476; PMID: 18941537
- Wang S, Raven JF, Koromilas AE. STAT1 represses Skp2 gene transcription to promote p27Kip1 stabilization in Ras-transformed cells. Mol Cancer Res 2010; 8:798 - 805; http://dx.doi.org/10.1158/1541-7786.MCR-10-0027; PMID: 20407011
- Kovacic B, Stoiber D, Moriggl R, Weisz E, Ott RG, Kreibich R, et al. STAT1 acts as a tumor promoter for leukemia development. Cancer Cell 2006; 10:77 - 87; http://dx.doi.org/10.1016/j.ccr.2006.05.025; PMID: 16843267
- Watson CJ. Stat transcription factors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia 2001; 6:115 - 27; http://dx.doi.org/10.1023/A:1009524817155; PMID: 11467447
- Widschwendter A, Tonko-Geymayer S, Welte T, Daxenbichler G, Marth C, Doppler W. Prognostic significance of signal transducer and activator of transcription 1 activation in breast cancer. Clin Cancer Res 2002; 8:3065 - 74; PMID: 12374673
- Yau C, Esserman L, Moore DH, Waldman F, Sninsky J, Benz CC. A multigene predictor of metastatic outcome in early stage hormone receptor-negative and triple-negative breast cancer. Breast Cancer Res 2010; 12:R85; http://dx.doi.org/10.1186/bcr2753; PMID: 20946665
- Klover PJ, Muller WJ, Robinson GW, Pfeiffer RM, Yamaji D, Hennighausen L. Loss of STAT1 from mouse mammary epithelium results in an increased Neu-induced tumor burden. Neoplasia 2010; 12:899 - 905; PMID: 21076615
- Raven JF, Williams V, Wang S, Tremblay ML, Muller WJ, Durbin JE, et al. Stat1 is a suppressor of ErbB2/Neu-mediated cellular transformation and mouse mammary gland tumor formation. Cell Cycle 2011; 10:794 - 804; http://dx.doi.org/10.4161/cc.10.5.14956; PMID: 21311224
- Chan SR, Vermi W, Luo J, Lucini L, Rickert C, Fowler AM, et al. STAT1-deficient mice spontaneously develop estrogen receptor α-positive luminal mammary carcinomas. Breast Cancer Res 2012; 14:R16; http://dx.doi.org/10.1186/bcr3100; PMID: 22264274
- Schneckenleithner C, Bago-Horvath Z, Dolznig H, Neugebauer N, Kollmann K, Kolbe T, et al. Putting the brakes on mammary tumorigenesis: loss of STAT1 predisposes to intraepithelial neoplasias. Oncotarget 2011; 2:1043 - 54; PMID: 22185785
- Ursini-Siegel J, Hardy WR, Zuo D, Lam SH, Sanguin-Gendreau V, Cardiff RD, et al. ShcA signalling is essential for tumour progression in mouse models of human breast cancer. EMBO J 2008; 27:910 - 20; http://dx.doi.org/10.1038/emboj.2008.22; PMID: 18273058
- Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J 1999; 18:2149 - 64; http://dx.doi.org/10.1093/emboj/18.8.2149; PMID: 10205169
- Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 1996; 84:443 - 50; http://dx.doi.org/10.1016/S0092-8674(00)81289-1; PMID: 8608598
- Han W, Carpenter RL, Cao X, Lo HW. STAT1 gene expression is enhanced by nuclear EGFR and HER2 via cooperation with STAT3. Mol Carcinog 2012; In press http://dx.doi.org/10.1002/mc.21936; PMID: 22693070
- Ursini-Siegel J. Beyond immune surveillance: Stat1 limits tumor growth in a cell-autonomous fashion. Cell Cycle 2011; 10:1348; http://dx.doi.org/10.4161/cc.10.9.15435; PMID: 21464617
- Tenoever BR, Ng SL, Chua MA, McWhirter SM, García-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science 2007; 315:1274 - 8; http://dx.doi.org/10.1126/science.1136567; PMID: 17332413
- Bonuccelli G, Castello-Cros R, Capozza F, Martinez-Outschoorn UE, Lin Z, Tsirigos A, et al. The milk protein α-casein functions as a tumor suppressor via activation of STAT1 signaling, effectively preventing breast cancer tumor growth and metastasis. Cell Cycle 2012; 11:3972 - 82; http://dx.doi.org/10.4161/cc.22227; PMID: 23047602
- Khodarev NN, Minn AJ, Efimova EV, Darga TE, Labay E, Beckett M, et al. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer Res 2007; 67:9214 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-07-1019; PMID: 17909027
- Khodarev NN, Roach P, Pitroda SP, Golden DW, Bhayani M, Shao MY, et al. STAT1 pathway mediates amplification of metastatic potential and resistance to therapy. PLoS One 2009; 4:e5821; http://dx.doi.org/10.1371/journal.pone.0005821; PMID: 19503789
- Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A 2008; 105:18490 - 5; http://dx.doi.org/10.1073/pnas.0809242105; PMID: 19001271
- Thomas M, Finnegan CE, Rogers KM, Purcell JW, Trimble A, Johnston PG, et al. STAT1: a modulator of chemotherapy-induced apoptosis. Cancer Res 2004; 64:8357 - 64; http://dx.doi.org/10.1158/0008-5472.CAN-04-1864; PMID: 15548705
- Harris LN, Yang L, Liotcheva V, Pauli S, Iglehart JD, Colvin OM, et al. Induction of topoisomerase II activity after ErbB2 activation is associated with a differential response to breast cancer chemotherapy. Clin Cancer Res 2001; 7:1497 - 504; PMID: 11410482
- Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, et al. c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 1994; 330:1260 - 6; http://dx.doi.org/10.1056/NEJM199405053301802; PMID: 7908410
- Pritchard KI, Shepherd LE, O’Malley FP, Andrulis IL, Tu D, Bramwell VH, et al, National Cancer Institute of Canada Clinical Trials Group. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 2006; 354:2103 - 11; http://dx.doi.org/10.1056/NEJMoa054504; PMID: 16707747
- Magkou C, Giannopoulou I, Theohari I, Fytou A, Rafailidis P, Nomikos A, et al. Prognostic significance of phosphorylated STAT-1 expression in premenopausal and postmenopausal patients with invasive breast cancer. Histopathology 2012; 60:1125 - 32; http://dx.doi.org/10.1111/j.1365-2559.2011.04143.x; PMID: 22320867
- Rajski M, Vogel B, Baty F, Rochlitz C, Buess M. Global gene expression analysis of the interaction between cancer cells and osteoblasts to predict bone metastasis in breast cancer. PLoS One 2012; 7:e29743; http://dx.doi.org/10.1371/journal.pone.0029743; PMID: 22235336