Abstract
Osteoblast differentiation is a critical step in the maintenance of bone homeostasis. Osteoblast differentiation is generally maintained by growth hormone (GH) and various other endocrine and autocrine/paracrine factors. JAK2-STAT5B pathway is a central axis in the mechanism of GH signaling. Similarly, the autocrine/paracrine signaling factor IGF-1 also mediates its effects through this pathway. Analysis on JAK2-STAT5B pathway showed its importance in the IGF-1/IGF-1R mediated regulation of gene expression and osteoblast differentiation. Persistent activation of STAT5B and inhibition of STAT5B degradation showed increased osteoblastic differentiation and STAT5B/Runx-2 activities. Conditional gene silencing studies showed the importance of the JAK2-STAT5B pathway in stimulation of other transcription factors and expression of various differentiation markers.
Introduction
Signal transducer and activator of transcription (STAT) was first identified two decades ago. From there the development happened in the field of STAT family of proteins, and its upstream regulator Janus activated kinases (JAKs) shows the importance of STATs in biological systems. Recent evidence suggests that STAT family of protein, especially STAT5B plays a pivotal role in growth hormone (GH) signaling, osteoblast differentiation and bone homeostasis.Citation1 Indeed, our in vitro studies showed that the enhancement of GH signaling using methylsulfone (MSM), a low molecular weight natural compound induced the osteoblast differentiation through persistent activation of JAK2-STAT5B pathways.Citation2 Different osteogenic markers were expressed in osteoblast like cells in response to JAK2-STAT5B signaling. Even then the exact role of JAK2-STAT5B signaling axis in osteoblast differentiation remains unclear. This paper focuses on the role of JAK2-STAT5B pathway in GH-dependent osteoblast differentiation.
Biological Functions of Osteoblast in Bone
Bone is a specialized connective tissue which supports the body, housing for vital organs, maintains blood calcium levels, support hematopoiesis and have several metabolic functions. Bone contains distinctive layers like outer shell called the cortex, and a hollow cavity filled with bone marrow. Different degrees of mineralization are observed in bone, which makes it harder and stronger. Even though bone is a living tissue containing blood vessels and various cell type (). Bone is continuously maintained and renewed by four different kinds of cells:
Figure 1. Different cells of bone maintenance and homeostasis. Osteoblasts are differentiated from mesenchymal cell precursors. The mature osteoblast then differentiated into osteocytes and lining cells. These constitutes most of bones cells and protect bone from mechanical damages.
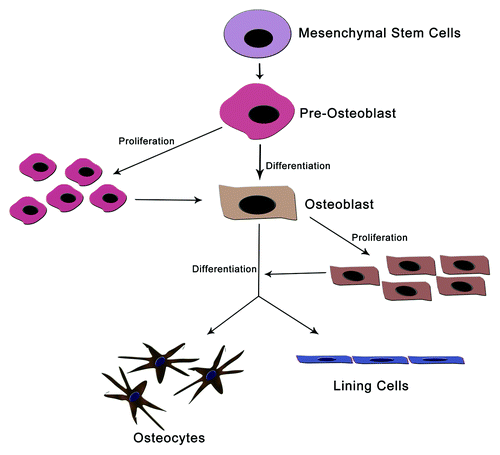
1) Osteoclasts: Osteoclasts are giant multinucleated cells located at the surface of bone. They degrade (resorb) bone tissue via local acidification and secretion of various proteases. Acidification is crucial to dissolve the mineral in bone, whereas the proteases degrade the proteins of the extracellular matrix.
2) Osteoblasts: Osteoblasts are derived from hematopoietic cells. They synthesize the organic matrix of bone by secretion of a wide variety of extracellular matrix proteins. This group of cells plays a critical role in the maintenance of bone health by increasing the bone mineralization process and controlling the resorption role of osteoclasts. Osteoblast at its terminal stage of life, either undergo a differentiation or an apoptotic death.
3) Osteocytes: Osteocytes are the terminal differentiation products of osteoblasts. These cells occur entrapped in the bone matrix produced by the cell itself. They have a morphology with long thin cytoplasmic processes. This morphology helps them to form a fine network of connections with other osteocytes, and with the osteoblasts located at the surface of the bone. Osteocytes are the most abundant cells in the bone and are believed to maintain the bone by sensing mechanical strains and bone damage.Citation3-Citation5
4) Lining cells: These are another group of bone cells derived from osteoblasts. Lining cells cover the bone surfaces and separate the bone surface from the bone marrow. However, the exact function of bone lining cells remains unclear.Citation6
This indicates that differentiation of osteoblasts is very much important for the maintenance of bone from mechanical strain and from other damages. Furthermore, increase the mineralization of bone and minimize the bone resorption.
Janus Kinases and Signal Transduction
JAKs are the family to PTKs with a molecular weight of 120–130 kDa, and consisting ~1150 amino acids.Citation7-Citation9 Four distinct members of the JAK family have been identified, namely, JAK1, JAK2, JAK3, and Tyk2. This family of proteins contains a tandem kinase and a pseudokinase domain. JAKs are expressed ubiquitous with an exemption of JAK3, which is expressed in hematopoietic cells, and vascular smooth muscles and endothelium.Citation10-Citation14
The individual role of JAK2 is too wide as it can regulate a broad range of signaling molecules and transcription factors. Upon different external signal, JAK2 acts on various substrates. Limited knowledge is available about JAK substrates until now. Even then the major substrate of JAK is the STAT family of transcription factors. Apart from this, Shc, Grb2, Vav, STAM, and SHP2 have been reported to interact with JAKs.Citation15-Citation18
Signal Transducers and Activators of Transcription (STATs) in Signal Transduction
The signal transducer and activator of transcription (STAT), first discovered in 1993 by James DarnellCitation19,Citation20 is a group of seven members denoted STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6, plays a central role in growth factors, prolactin and various other cytokine signaling.Citation20,Citation21 STAT signaling axis controls a remarkable variety of biological responses, including development, differentiation, cell proliferation and survival.Citation22 STAT5 originally found in mammary gland and was called as a mammary gland factor.Citation23 Later in 1995, STAT5B was discovered and STAT5 renamed to STAT5A.Citation24 Recent studies found that STAT5B is expressed in many cell types, including osteoblasts. The recognition of JAK2-STAT5B-IGF-1 signaling axis helped the scientific community to understand the signaling of growth hormones and other endocrine and paracrine/autocrine hormones, which use IGF-1 for signaling. It has been clear that, extracellular ligand-binding receptor-associated cytoplasmic tyrosine kinases belonging to the JAK family are activated upon cytokine or growth hormone binding. Upon activation, the JAKs cross phosphorylate each other and their associated membrane receptors. Additionally, the intimate relationship between JAK2 and STAT5B has made it difficult to attribute discrete actions to each molecule in osteoblast differentiation.
Growth Hormone and Osteoblast Differentiation
GH promotes longitudinal bone growth and regulates multiple cellular functions in humans and animals.Citation25 GH signals by binding to GH receptor (GHR) to activate the tyrosine kinase, JAK2, which in turn tyrosine phosphorylates GHR and downstream STAT5 pathways, thereby regulating expression of genes, including IGF-1. Orlando et al., reported that GH signaling via the JAK2-STAT5 pathway can be prolonged by 1,25(OH)2D3 in UMR-106 osteoblast like cells.Citation26 Prolonged activation of JAK2-STAT5B leads to the DNA binding and transcriptional functions of STAT5B. Recently, we found that methylsulfone induced expression and phosphorylation of STAT5B can modulate the expression of GHR in osteoblastic MC3T3-E1 and in UMR-106 cells.Citation2
JAK2-STAT5B Pathway in IGF-1/IGF-1R Signaling Axis
GH signals both directly and via IGF-1, which signals by activating the IGF-1 receptor (IGF-1R). IGF-1 is reported as the critical mediator of bone growth.Citation27 Systemic IGF-1 are synthesized primarily in the liver, where its synthesis is GH-dependent; IGF-1 is also produced in osteoblasts, where it acts locally as an autocrine/paracrine growth factor under the control of multiple hormones.Citation28,Citation29 Recently, we reported that, methylsulfone, a natural organic sulfur containing compound can stimulate JAK2-STAT5B signaling and can mimic the GH signaling through JAK2-STAT5B pathway. In our study, we detected that STAT5B has transcriptional promoter activity in IGF-1 as well as in IGF-1R genes in UMR-106 cells. Similar DNA binding activities were seen in UMR-106 cells treated with 1,25(OH)2D3.Citation26
IGF-1R is a cell surface receptor that contains intrinsic tyrosine kinase activity within its intracellular domain. Recent studies in our lab reported that silencing STAT5B using specific siRNA showed a decline in the expression of IGF-1R in osteoblastic MC3T3-E1Citation30 and in C3H10T1/2 cells.Citation2 Studies also proved that STAT5B has a DNA binding domain in IGF-1R and this binding is stimulated by the phosphorylation of STAT5B by JAK2. The binding of STAT5B to the GAS-DNA binding domain of IGF-1R increases the relative expression of IGF-1R in UMR-106 cells.Citation2 When these cells treated with a JAK2 inhibitor AG490, STAT5B induced expression of IGF-1R seems inhibited. Similarly, the deletion of IGF-1R from primary osteoblast showed a reduction in the tyrosine phosphorylation of STAT5, and STAT5-mediated GH signaling.Citation31 This gives an idea about the positive feedback loop of control between STAT5 and IGF-1R. However IGF-1R tyrosine kinase inhibitor did not prevent the GH induced STAT5 activation whereas it inhibited the IGF-1 induced IGF-1R signaling.
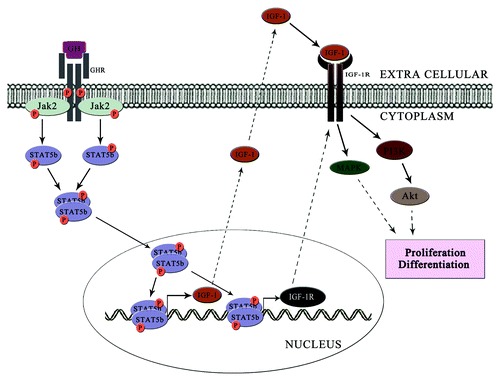
GH signaling in osteoblast can be explained in two phases. In the first phase, GH binds to the GHR leading to the receptor autophosphorylation and JAK2 phosphorylation. This JAK2 phosphorylation in turn activates the STAT5B signaling pathway and trigger the expression of genes regulated by STAT5B. IGF-1 and IGF-1R are the prominent downstream targets of STAT5B. The synthesized IGF-1 is secreated out from the cell, and it will start operating the autocrine loop of signaling. Meanwhile, the synthesized IGF-1R translocate to the membrane and transmits the signals from IGF-1, which constitutes the second phase of GH signaling ().
Figure 2. GH transmits the signal in two phases. In the first phase (represented as dark arrow), GH binds to the GHR and triggers the JAK2-STAT5B signaling axis. This leads to the transcription of IGF-1 and IGF-1R. These then mediates the second phase (represented by dotted arrows) of signal transduction. Here IGF-1 binds to IGF-1R and triggers the MAPK pathway or PI3K/Akt pathway and induces osteoblast proliferation and differentiation.
JAK2-STAT5B and BMPs
The bone morphogenic proteins (BMPs) are the crucial intermediates of osteoblast differentiation through paracrine signaling. JAK2-STAT5B pathway can modulate the morphology of the bone growth by altering the bone morphogenic proteins in the osteoblastic and pre-osteoblastic cell lines. Recently, we found that, inhibiting the JAK2 using AG490 and silencing of STAT5B using specific siRNA inhibits the expression of BMP-7 in MC3T3-E1 cells.Citation30 This clearly tells the role of JAK2-STAT5B in the expression of BMP-7. The role of BMP-7 in osteoblast cells is well studied.Citation32 One of the most important targets of BMPs is Runx-2.Citation33 Hypothetically, JAK2-STAT5B pathway can impart a direct or indirect role in this crucial transcription factor for osteoblastogenesis. However, this mechanism has to be clarified with sufficient experiments.
JAK2-STAT5B Pathway and Runx-2
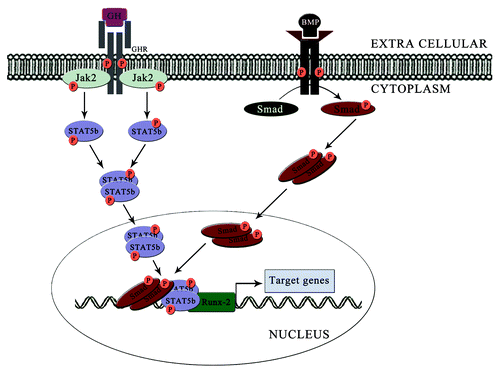
Runx-2 is a crucial transcription factor in the osteoblast differentiation. There are studies showing that the STAT5 can modulate the activities of Runx-2. Panos et al. showed that GH stimulates JAK2-STAT5 in Saos-2 human osteoblastic cells.Citation25 In this Saos-2 GH induced a transient tyrosine phosphorylation in STAT3 as well as in STAT5. They also found that, GH can regulate the role of Runx in Saos-2 cell lines. STAT5B siRNA transfected C3H10T1/2 showed a statistically significant decrease in expression of Runx-2.Citation2 In the same study, chemically induced increase in the expression of STAT5B showed elevated level of Runx-2 and increased osteoblast differentiation. Another study conducted on the role of Runx proteins in osteoblast differentiation, it is found that Runx-2 alone cannot induce osteoblastic differentiation. They suggest that smad complexes and some other proteins together with Runx-2 induces the osteoblast differentiation.Citation34 Degradation studies conducted by François-Xavier et al. on STAT5 using c-Cbl showed that silencing of c-Cbl can increase the interaction of STAT5-Runx-2 in mesenchymal stem cells. STAT5 forms a functional complex with Runx-2 leading to the increase in transcriptional activity of Runx-2 and expression of genes involved in osteoblast differentiation.Citation35 This work shows the ability to STAT5B in regulating the activity of Runx-2 for osteoblast differentiation process ().
Figure 3. Regulation of Runx-2 activity by combined effect of STAT5B and Smad complexes. Induction of STAT5B from GH signaling leads to the formation of STAT5B/Runx-2 complex. Similarly, the Smad complexes induced by the action of BMP will bind with the Runx-2 and form Smad/STAT5B/Runx-2 complex and induce the transcription of genes responsible for osteoblast differentiation.
Other Targets of JAK2-STAT5B in Osteoblasts
Tbx3 (T-box3) is a transcription factor known for its transcription regulation. Tbx is known to express in bone, and its expression is regulated via GH. Osteoblast proliferation is regulated by Tbx3 and during osteoblast differentiation, its expression increases.Citation36,Citation37 Blocking Tbx3 resulted in inhibition of stromal cell differentiation into osteoblasts showing the crucial role of Tbx3 in osteoblast differentiation.Citation38 Govoni et al. also showed that, pre-treatment of MC3T3-E1 cells with STAT5B inhibitor TNF-α abolished the GH-mediated Tbx3 expression. This suggests that, JAK2-STAT5B pathway is involved in the expression of Tbx3 in MC3T3-E1 osteoblastic cells. Even though overexpression of Tbx3 suppresses the osteoblastic differentiation and maintains the homeostasis through suppressing osterix and Runx2.
Additionally, this study showed STAT5B can directly or indirectly affect various osteogenic markers like, ALP, BSP, OCN, OPN, and Osterix. More studies need to elucidate the role of the JAK2-STAT5B pathway in the regulation of all these osteogenic markers.
Conclusion
The importance of the JAK2-STAT5B pathway in the microenvironment of osteoblastic cells contributes to the increasing knowledge of osteoblast differentiation. Osteoblast is highly affected by the GH, which signals through JAK2-STAT5B pathway. The secrets in the regulation of different signaling pathways and their interrelationships with JAK2-STAT5B pathway should be unraveled in osteoblasts. We expect the new information may help in developing drugs targeted to JAK2-STAT5B modulation, and it will help patients with various bone disorders, deformities, and growth disorders.
Acknowledgments
This work was supported by a grant from the Next generation BioGreen 21 Program (PJ0081062011), Rural Development Administration, and was partially supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0004568), Republic of Korea.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Zhu T, Goh EL, Graichen R, Ling L, Lobie PE. Signal transduction via the growth hormone receptor. Cell Signal 2001; 13:599 - 616; http://dx.doi.org/10.1016/S0898-6568(01)00186-3; PMID: 11495718
- Joung YH, Lim EJ, Darvin P, Chung SC, Jang JW, Do Park K, et al. MSM enhances GH signaling via the Jak2/STAT5b pathway in osteoblast-like cells and osteoblast differentiation through the activation of STAT5b in MSCs. PLoS One 2012; 7:e47477; http://dx.doi.org/10.1371/journal.pone.0047477; PMID: 23071812
- Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med 2006; 354:2250 - 61; http://dx.doi.org/10.1056/NEJMra053077; PMID: 16723616
- Burger EH, Klein-Nulen J. Responses of bone cells to biomechanical forces in vitro.. Adv Dent Res 1999; 13:93 - 8; http://dx.doi.org/10.1177/08959374990130012201; PMID: 11276754
- Noble B. Bone microdamage and cell apoptosis. Eur Cell Mater 2003; 6:46 - 55, 55; PMID: 14710370
- Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res 2001; 16:1583 - 5; http://dx.doi.org/10.1359/jbmr.2001.16.9.1583; PMID: 11547827
- Rane SG, Reddy EP. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene 1994; 9:2415 - 23; PMID: 7518579
- Gurniak CB, Berg LJ. Murine JAK3 is preferentially expressed in hematopoietic tissues and lymphocyte precursor cells. Blood 1996; 87:3151 - 60; PMID: 8605329
- Lai KS, Jin Y, Graham DK, Witthuhn BA, Ihle JN, Liu ET. A kinase-deficient splice variant of the human JAK3 is expressed in hematopoietic and epithelial cancer cells. J Biol Chem 1995; 270:25028 - 36; http://dx.doi.org/10.1074/jbc.270.42.25028; PMID: 7559633
- Kawamura M, McVicar DW, Johnston JA, Blake TB, Chen YQ, Lal BK, et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci U S A 1994; 91:6374 - 8; http://dx.doi.org/10.1073/pnas.91.14.6374; PMID: 8022790
- Sharfe N, Dadi HK, O’Shea JJ, Roifman CM. Jak3 activation in human lymphocyte precursor cells. Clin Exp Immunol 1997; 108:552 - 6; http://dx.doi.org/10.1046/j.1365-2249.1997.4001304.x; PMID: 9182906
- Tortolani PJ, Lal BK, Riva A, Johnston JA, Chen YQ, Reaman GH, et al. Regulation of JAK3 expression and activation in human B cells and B cell malignancies. J Immunol 1995; 155:5220 - 6; PMID: 7594533
- Musso T, Johnston JA, Linnekin D, Varesio L, Rowe TK, O’Shea JJ, et al. Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7. J Exp Med 1995; 181:1425 - 31; http://dx.doi.org/10.1084/jem.181.4.1425; PMID: 7535338
- Verbsky JW, Bach EA, Fang YF, Yang L, Randolph DA, Fields LE. Expression of Janus kinase 3 in human endothelial and other non-lymphoid and non-myeloid cells. J Biol Chem 1996; 271:13976 - 80; http://dx.doi.org/10.1074/jbc.271.24.13976; PMID: 8662778
- Yin T, Shen R, Feng GS, Yang YC. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J Biol Chem 1997; 272:1032 - 7; http://dx.doi.org/10.1074/jbc.272.2.1032; PMID: 8995399
- Chauhan D, Kharbanda SM, Ogata A, Urashima M, Frank D, Malik N, et al. Oncostatin M induces association of Grb2 with Janus kinase JAK2 in multiple myeloma cells. J Exp Med 1995; 182:1801 - 6; http://dx.doi.org/10.1084/jem.182.6.1801; PMID: 7500025
- Matsuguchi T, Inhorn RC, Carlesso N, Xu G, Druker B, Griffin JD. Tyrosine phosphorylation of p95Vav in myeloid cells is regulated by GM-CSF, IL-3 and steel factor and is constitutively increased by p210BCR/ABL. EMBO J 1995; 14:257 - 65; PMID: 7530656
- Takeshita T, Arita T, Higuchi M, Asao H, Endo K, Kuroda H, et al. STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction. Immunity 1997; 6:449 - 57; http://dx.doi.org/10.1016/S1074-7613(00)80288-5; PMID: 9133424
- Shuai K, Stark GR, Kerr IM, Darnell JE Jr.. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-γ. Science 1993; 261:1744 - 6; http://dx.doi.org/10.1126/science.7690989; PMID: 7690989
- Levy DE, Darnell JE Jr.. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3:651 - 62; http://dx.doi.org/10.1038/nrm909; PMID: 12209125
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci 2006; 1091:151 - 69; http://dx.doi.org/10.1196/annals.1378.063; PMID: 17341611
- Jove R. Preface: STAT signaling. Oncogene 2000; 19:2466 - 7; http://dx.doi.org/10.1038/sj.onc.1203549; PMID: 10851044
- Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev 2008; 22:711 - 21; http://dx.doi.org/10.1101/gad.1643908; PMID: 18347089
- Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A 1995; 92:8831 - 5; http://dx.doi.org/10.1073/pnas.92.19.8831; PMID: 7568026
- Ziros PG, Georgakopoulos T, Habeos I, Basdra EK, Papavassiliou AG. Growth hormone attenuates the transcriptional activity of Runx2 by facilitating its physical association with Stat3β. J Bone Miner Res 2004; 19:1892 - 904; http://dx.doi.org/10.1359/JBMR.040701; PMID: 15476590
- Morales O, Faulds MH, Lindgren UJ, Haldosén LA. 1α,25-dihydroxyvitamin D3 inhibits GH-induced expression of SOCS-3 and CIS and prolongs growth hormone signaling via the Janus kinase (JAK2)/signal transducers and activators of transcription (STAT5) system in osteoblast-like cells. J Biol Chem 2002; 277:34879 - 84; http://dx.doi.org/10.1074/jbc.M204819200; PMID: 12107179
- Goldspink G. Loss of muscle strength during aging studied at the gene level. Rejuvenation Res 2007; 10:397 - 405; http://dx.doi.org/10.1089/rej.2007.0597; PMID: 17822355
- Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des 2007; 13:663 - 9; http://dx.doi.org/10.2174/138161207780249146; PMID: 17346182
- Rosen CJ. Insulin-like growth factor I and bone mineral density: experience from animal models and human observational studies. Best Pract Res Clin Endocrinol Metab 2004; 18:423 - 35; http://dx.doi.org/10.1016/j.beem.2004.02.007; PMID: 15261847
- Joung YH, Lim EJ, Pramod D, Chung SC, Jang JW, et al. HSE enhances BMP7 and GH signaling through the activation of Jak2/STAT5B in osteoblastic MC3T3-E1 cells. Mol Med Rep 2013; In press
- Gan Y, Zhang Y, Digirolamo DJ, Jiang J, Wang X, Cao X, et al. Deletion of IGF-I receptor (IGF-IR) in primary osteoblasts reduces GH-induced STAT5 signaling. Mol Endocrinol 2010; 24:644 - 56; http://dx.doi.org/10.1210/me.2009-0357; PMID: 20133448
- Lavery K, Hawley S, Swain P, Rooney R, Falb D, Alaoui-Ismaili MH. New insights into BMP-7 mediated osteoblastic differentiation of primary human mesenchymal stem cells. Bone 2009; 45:27 - 41; http://dx.doi.org/10.1016/j.bone.2009.03.656; PMID: 19306956
- Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem 2011; 112:3491 - 501; http://dx.doi.org/10.1002/jcb.23287; PMID: 21793042
- Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene 2004; 23:4232 - 7; http://dx.doi.org/10.1038/sj.onc.1207131; PMID: 15156178
- Dieudonne FX, Severe N, Biosse-Duplan M, Weng JJ, Su Y, Marie PJ. Promotion of Osteoblast Differentiation in Mesenchymal Cells Through Cbl-Mediated Control of STAT5 Activity. Stem Cells 2013; In press http://dx.doi.org/10.1002/stem.1380; PMID: 23533197
- Govoni KE, Lee SK, Chadwick RB, Yu H, Kasukawa Y, Baylink DJ, et al. Whole genome microarray analysis of growth hormone-induced gene expression in bone: T-box3, a novel transcription factor, regulates osteoblast proliferation. Am J Physiol Endocrinol Metab 2006; 291:E128 - 36; http://dx.doi.org/10.1152/ajpendo.00592.2005; PMID: 16464905
- Govoni KE, Linares GR, Chen ST, Pourteymoor S, Mohan S. T-box 3 negatively regulates osteoblast differentiation by inhibiting expression of osterix and runx2. J Cell Biochem 2009; 106:482 - 90; http://dx.doi.org/10.1002/jcb.22035; PMID: 19115250
- Lee HS, Cho HH, Kim HK, Bae YC, Baik HS, Jung JS. Tbx3, a transcriptional factor, involves in proliferation and osteogenic differentiation of human adipose stromal cells. Mol Cell Biochem 2007; 296:129 - 36; http://dx.doi.org/10.1007/s11010-006-9306-4; PMID: 16955224