 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aggregation compromises the safety and efficacy of therapeutic proteins. According to the manufacturer, the therapeutic immunoglobulin trastuzumab (Herceptin®) should be diluted in 0.9% sodium chloride before administration. Dilution in 5% dextrose solutions is prohibited. The reason for the interdiction is not mentioned in the Food and Drug Administration (FDA) documentation, but the European Medicines Agency (EMEA) Summary of Product Characteristics states that dilution of trastuzumab in dextrose solutions results in protein aggregation. In this paper, asymmetrical flow field-flow fractionation (FFF), fluorescence spectroscopy, fluorescence microscopy and transmission electron microscopy (TEM) have been used to characterize trastuzumab samples diluted in 0.9% sodium chloride, a stable infusion solution, as well as in 5% dextrose (a solution prone to aggregation). When trastuzumab samples were injected in the FFF channel using a standard separation method, no difference could be seen between trastuzumab diluted in sodium chloride and trastuzumab diluted in dextrose. However, during FFF measurements made with appropriate protocols, aggregates were detected in 5% dextrose. The parameters enabling the detection of reversible trastuzumab aggregates are described. Aggregates could also be documented by fluorescence microscopy and TEM. Fluorescence spectroscopy data were indicative of conformational changes consistent with increased aggregation and adsorption to surfaces. The analytical methods presented in this study were able to detect and characterize trastuzumab aggregates.
Introduction
During storage and before administration, therapeutic proteins are subject to degradation by many factors such as deamidation, racemization, isomerization, oxidation and aggregation.Citation1,Citation2 Stabilization of protein pharmaceuticals for long-term storage is often achieved by lyophilization.Citation3 Special precautions should be taken to ensure the integrity of the product during reconstitution and until administration. The presence of “seeds” in the reconstituted solution can lead to the formation of visible particles.Citation4 To avoid aggregate formation, the nature of the solutions used for reconstitution and for dilution before administration must be carefully chosen. Both the excipients used during the lyophilization process and the excipients used during the reconstitution process should ensure the stability of the protein. The reconstitution process in itself can also influence aggregation. Reconstitution instructions for therapeutic immunoglobulins often state that shaking should be avoided and in several cases (e.g., trastuzumab, infliximab, palivizumab and efalizumab) state that the solvent should be added slowly. A slow dissolution process has been shown to decrease the aggregate content of a protein after reconstitution.Citation5 Shaking causes foaming if detergents are present in the formulation, hampering a precise dosage of the drug. Furthermore, shaking can induce aggregation by increasing the water-air interface and by inducing mechanical stress.Citation6,Citation7 As underlined in the Package Insert,Citation8 trastuzumab (Herceptin®) may be sensitive to mechanical stress induced by agitation or rapid expulsion from a syringe.
Inappropriate protein handling can thus occur at several stages preceding administration, which can result in aggregation, further leading to reduced efficacy and potential side effects.Citation9–Citation11 Analytical techniques should be able to detect the changes in the aggregation state of therapeutic proteins resulting from mishandling. In the present paper, a combination of analytical techniques was evaluated on trastuzumab, a therapeutic immunoglobulin whose sales value is ranked among the top biotech drugs.Citation12,Citation13 The FDA-approved Package InsertCitation8 states that after reconstitution, trastuzumab must be diluted in an infusion bag containing 0.9% sodium chloride. The use of 5% dextrose is prohibited without providing further details. The explanation is however available in the Summary of Product Characteristics approved by the EMEA,Citation14 which states that dextrose solutions cause aggregation of the protein. Asymmetrical flow field-flow fractionation (FFF), fluorescence spectroscopy, fluorescence microscopy and transmission electron microscopy (TEM) measurements were performed on trastuzumab diluted in 0.9% sodium chloride or in 5% dextrose, a solution causing aggregation. Our data show that if the analytical conditions are carefully chosen, the methods presented in this paper allow the detection and characterization of trastuzumab aggregates in 5% dextrose.
Results
Investigation of trastuzumab aggregation by FFF.
Trastuzumab was diluted in 0.9% sodium chloride or 5% dextrose (final concentration 1.28 mg/ml) and different FFF experimental conditions were tested to investigate protein aggregation. Characterization was performed on both solutions within their published lifetime of 24 hours after dilution. When trastuzumab samples were injected in the FFF channel using a standard separation method (, Method 1) and with 0.9% sodium chloride as running buffer (carrier liquid), no difference could be seen between trastuzumab diluted in sodium chloride and diluted in dextrose (). The main peak had a molecular weight of 160 kDa. A limited number of small aggregates, mostly dimers, could be detected after 13 minutes of elution: 0.69% for trastuzumab diluted in 0.9% sodium chloride (NaCl-trastuzumab) and 0.64% for trastuzumab diluted in 5% dextrose (dextrose-trastuzumab). Based on the data presented in , one could conclude that no significant aggregates were present in dextrose-trastuzumab solutions.
According to the Summary of Product Characteristics,Citation14 dextrose-trastuzumab solutions are prone to aggregation. The absence of aggregates in the FFF runs was further investigated by injecting the samples in the system without applying any focussing flow or cross-flow (, Method 2). The samples were in limited contact with the channel membrane. As no focussing or cross-flow was applied, the separation did not take place, and the entire sample eluted in one broad peak within two minutes. Consequently, the light scattering analysis only provided the mean size of the sample, strongly influenced by the larger species.Citation19 shows that without separation, high molecular weights were observed after 2 minutes of elution. Such high observed molecular weights can be attributed to dust present in the sample (the solutions were not filtered), or to the abrupt change in solution properties resulting from the relatively high concentration of the eluted sample. More relevant is the light scattering signal observed after 2.5 minutes of elution. In the case of a mainly monomeric solution (), the light scattering signal at 2.5–3.5 minutes was flat and indicated the molecular weight of the monomer. The presence of high molecular weight aggregates resulted in higher and noisier molecular weight data after 2.5 minutes of elution ( and C) When analyzing NaCl-trastuzumab with 0.9% NaCl as the running buffer, the solutions appeared mainly monomeric, with a molecular weight around 160 kDa (). The UV signals (solid lines) obtained from 3 or 4 separate injections showed similar shape and area under the curve. When analyzing dextrose-trastuzumab with 5% dextrose as the running buffer (), the sample recovery was variable and lower than in sodium chloride, as indicated by the UV signals from the 3 injections (solid lines) being lower in intensity and less reproducible compared to . The variability of the recovery can be rationalized as being due to higher adsorption of the sample to the FFF system. During the analysis of dextrosetrastuzumab, FFF UV signal baselines never came back to zero and molecular weight was variable and several orders of magnitude higher than 160 kDa: aggregates were detected (). When NaCl-trastuzumab was analysed with 5% dextrose as the running buffer, the sample recovery was also variable and molecular weights were higher than 160 kDa indicating that aggregation took place immediately in the FFF channel (). On the contrary, when dextrose-trastuzumab was analysed with 0.9% NaCl as the running buffer, aggregates partially disappeared and even though the apparent molecular weight was slightly higher than expected, the recovery was good, reproducible and baselines came back to zero (). The disappearance of trastuzumab aggregates in 0.9% NaCl is indicative of reversible aggregation. Trastuzumab aggregates can be overlooked if the FFF analysis of dextrose-trastuzumab is performed in 0.9% NaCl.
After the analysis of aggregated trastuzumab (), UV and light scattering signals were monitored during a thorough rinsing of the FFF channel, obtained by switching repeatedly between the elution and focus modes. The rinsing procedure was able to detach protein material adsorbed to the FFF system and revealed the presence of larger aggregates (). The adsorption of trastuzumab to the FFF system in 5% dextrose has not allowed a more detailed characterization of aggregates formed in dextrose solutions by FFF, but the strong, rapid and reversible aggregation of trastuzumab molecules upon dilution in dextrose solutions could be detected.
Characterization of trastuzumab solutions by fluorescence spectroscopy.
ANS fluorescence can be used to probe changes in the tertiary structure of proteins.Citation20 The increase in the ANS fluorescence concomitant with a shift of the ANS fluorescence to shorter wavelengths () indicate a strong binding of ANS to trastuzumab diluted in dextrose. The higher anisotropy () and longer ANS lifetime () confirm the stronger binding of ANS to trastuzumab diluted in 5% dextrose compared to trastuzumab diluted in 0.9% NaCl.Citation21
Characterization of trastuzumab solutions by fluorescence microscopy.
Nile Red has been shown to stain protein aggregates, allowing their detection by fluorescence microscopy.Citation22 Trastuzumab solutions diluted both in 0.9% NaCl and in 5% dextrose were analysed by fluorescence microscopy. The final concentration was 1.28 mg/ml, as in the FFF measurements. Particle counting was performed on both solutions within their published lifetime of 24 hours after dilution. In 0.9% NaCl, trastuzumab aggregates were <10 µm, whereas in 5% dextrose, particles were often >10 µm (). In 0.9% NaCl, trastuzumab solutions contained 29 particles/microliter (n = 768), whereas in 5% dextrose, trastuzumab solutions contained 55 particles/microliter (n = 1498, where n indicates the number of particles counted). Observation of the samples by phase contrast microscopy in the absence of Nile Red also allowed the visualization of trastuzumab particles.
Nile Red was dissolved in ethanol prior to addition in the protein solution (see Materials and Methods). In order to check that ethanol did not trigger trastuzumab aggregation, trastuzumab solutions were analysed before and after ethanol addition. The addition of ethanol alone to trastuzumab solutions in 0.9% NaCl as well as in 5% dextrose did not influence the light scattering signal, even after 2 hours incubation at room temperature (data not shown). Measurements were performed immediately after Nile Red staining, in order to minimize Nile Red-induced aggregation.
Characterization of trastuzumab solutions by electron microscopy.
In order to detect the presence of aggregates, trastuzumab solutions diluted both in 0.9% NaCl and in 5% dextrose have been observed by transmission electron microscopy (TEM). The final concentration was 1.28 mg/ml. shows that no aggregates could be seen in trastuzumab diluted in 0.9% NaCl (NaCl-trastuzumab, ) whereas trastuzumab diluted in 5% dextrose showed many aggregates which appeared as black dots (dextrose-trastuzumab, ). Higher-magnification photomicrographs confirmed that no aggregates were present in NaCl-trastuzumab () and indicated that dextrose-trastuzumab formed aggregates of 100–200 nm in diameter (). Dextrose-trastuzumab aggregates appeared amorphous, without fibrillar structure (). Control photomicrographs of 0.9% NaCl and 5% dextrose alone were taken and show no particles ().
Discussion
Several methods that allow the detection and characterization of protein aggregates have already been reviewed.Citation23 The response of selected methods was compared in the present paper, for the analysis of aggregates formed by a deliberately mishandled therapeutic protein: trastuzumab diluted in 5% dextrose. When a standard FFF separation method was performed using 0.9% sodium chloride as the running buffer (), no differences could be seen between trastuzumab diluted in sodium chloride (a stable solution) and trastuzumab diluted in dextrose (an aggregated solution). During FFF measurements, the separation step can represent a degrading environment for the protein sample. The sample is in prolonged contact with the running buffer, which can influence the aggregation or de-aggregation process. The focus- and cross-flow applied during separation induce a shear stress that may disrupt protein aggregates. During the focussing step, protein samples can be adsorbed on the membrane that forms the bottom of the FFF channel. The FFF operating conditions and methodology should be designed to identify a potential degradation of the sample during analysis.
Our data show that in the presence of reversible aggregation, the use of a relevant running buffer during separation was crucial for the characterization of the protein sample: the use of a running buffer similar to the formulation buffer has revealed the presence of aggregates in dextrose solutions (). Analysis of dextrose-aggregated trastuzumab in a running buffer other than 5% dextrose (such as in ) can lead to the false conclusion that no aggregates were present in the solution. The influence of the running buffer on protein aggregation should be evaluated during FFF measurements, as well as in other separative techniques such as size-exclusion chromatography, in order to allow the detection of reversible aggregates.
As shown in and in , the presence of aggregates can be detected by FFF analysis even without separation. Factors considered for the detection of aggregates were the apparent molecular weight after three minutes of elution, the level and variability of sample recovery (as indicated by the UV signal) and the quality of the baselines. The return of baselines to zero is a significant parameter; high baselines should not be misinterpreted as instrumental errors. The decreased FFF recovery ( and C) indicated that dextrose-trastuzumab aggregates tend to stick to surfaces. Only a strong rinsing procedure detached the aggregates (). The versatility of FFF and its sensitivity to protein aggregates in a large variety of conditions can allow it to serve as an orthogonal method to size-exclusion chromatography.Citation24,Citation25
Protein aggregation is generally concomitant with a change in the protein's conformation,Citation2 which can modify the solvent exposure of hydrophobic residues. Binding of ANS to trastuzumab was stronger in the presence of 5% dextrose: ANS exhibited a stronger and blue-shifted fluorescence (). The decreased mobility of ANS (higher anisotropy, ) can be explained by the binding to trastuzumab.Citation21 The binding of ANS to trastuzumab is caused by a change of molecular conformation when diluted in 5% dextrose: hydrophobic residues were more exposed, consistent with increased adsorption to surfaces encountered in the FFF measurements.
Fluorescence microscopy confirmed the data obtained by FFF and fluorescence spectroscopy (). The presence of particulate matter was higher in dextrose solutions than in NaCl solutions, as indicated by the number of particles per microliter (55 and 29, respectively). Despite the qualitative nature of particle counting by fluorescence microscopy, interesting observations can be made with regards to the size and shape of protein particles. The particles observed in sodium chloride were systematically <10 µm, which is too small to be taken into account by the European and US Pharmacopoeia in the particulate contamination test. On the contrary, trastuzumab particles formed in dextrose solutions were larger and present in higher number. Trastuzumab solutions in NaCl and in dextrose could be compared by fluorescence microscopy and the solutions in dextrose were shown to contain more aggregates. Transmission electron microscopy confirmed the presence of aggregates in dextrose solutions ( and ). The fluorescence and electron microscopy methods used on trastuzumab samples detect aggregates of different, not overlapping sizes. The micron-sized aggregates detected by fluorescence microscopy would fill the whole field of view of an electron photomicrograph. Trastuzumab was aggregated in dextrose solutions across a wide range of particle sizes, as indicated by fluorescence and electron microscopy.
Trastuzumab aggregation in NaCl as well as dextrose solutions was assessed by a panel of analytical methods, presented in Table 3. FFF, fluorescence microscopy (after staining with Nile Red) and TEM showed that trastuzumab was more aggregated in dextrose solutions than in NaCl solutions. Fluorescence spectroscopy indicated a higher exposure of hydrophobic residues in dextrose-trastuzumab, consistent with the observed aggregation and increased adsorption to the FFF system. The level of aggregation in NaCl solutions was extremely low and only a few small particles could be visualized by fluorescence microscopy. Dextrose-trastuzumab aggregates were shown to be sticky, which limited their characterization by FFF. Trastuzumab aggregation was shown to be concomitant with conformational changes. The selection of methods used in the present paper successfully allowed the detection of reversible trastuzumab aggregates. In conclusion, a comparison of protein solutions (e.g., before and after lyophilization or before and after stability testing) can efficiently be performed during drug development by the methods presented in the present paper.
Materials and Methods
Materials.
Trastuzumab (Herceptin®) is an IgG1ϰ recombinant humanized monoclonal antibody co-marketed by Genentech, Inc., South San Francisco, USA, and Roche AG, Basel, Switzerland. Trastuzumab was purchased from a local pharmacy. An absorbance of 1.528 for a 1 mg/ml solution in a 1-cm cell was determined by UV spectroscopy at 280 nm, based upon a concentration of 21 mg/ml in the final product. The UV measurements were performed at 25°C with a temperature-controlled Cintra 40 spectrophotometer (GBC, Melbourne, Australia). Nile Red (9-diethylamino-5H-benzo[α]phenoxazine-5-one) was purchased from Sigma (Buchs, Switzerland). Nile Red was dissolved in ethanol, to produce a 100 µM stock solution, which was stored at 4°C, protected from light. A stock solution of 4 mM 1-anilinonaphthalene-8-sulfonic acid (ANS) in ethanol was prepared and stored at 4°C, protected from light. All water used in the experiments was deionized by a Milli-Q academic system (Millipore, Billerica, USA).
Asymmetrical flow field-flow fractionation (FFF).
Fractionation of the protein samples was performed in a trapezoidal channel, 26.5 cm in length and 350 µm in height, connected to an Eclipse F system (Wyatt Technology Europe, Dernbach, Germany). The bottom of the channel was lined with a poly(ether sulfone) membrane with a 10 kDa cut-off (Microdyn-Nadir GmbH, Wiesbaden, Germany). The quantity of trastuzumab injected into the system was 6.4 µg in 5 µl. The running buffer was 0.9% NaCl or 5% dextrose in water. The channel flow was set to 1 ml/min and the injection flow to 0.2 ml/min. The separation methods used in this paper are presented in . Each run was preceded by a conditioning step and if a cross-flow was applied, followed by a rinsing step with the cross-flow set to 0 ml/min.
A Dawn EOS multi-angle light scattering detector (Wyatt Technology, Santa Barbara, USA) and a UV detector (Agilent Technologies Schweiz AG, Basel, Switzerland) were coupled in-line with the FFF channel. The light scattering detector was equipped with a GaAs laser (wavelength: 690 nm) and eighteen detectors. Scattered light was collected at defined angles between 14 and 163 degrees. Sample concentration was determined by UV absorbance at 280 nm. Data were collected and analysed with the Astra version 4.90.08 software. A refractive index increment (dn/dc) of 0.185 ml/g was considered to be a sufficient approximation.Citation15 A second virial coefficient of 1 10−4 mol ml/g2 was used, assuming small repulsive forces between the proteins as seen in low ionic strength protein solutions.Citation16–Citation17 The use of theoretical values for dn/dc and second virial coefficient do not compromise the ability of FFF to distinguish monomers from aggregates. The results are reported in terms of molecular weight (Daltons).
Fluorescence measurements.
The steady-state fluorescence and fluorescence anisotropy measurements were performed with a Fluoromax spectrofluorometer (Spex, Stanmore, UK) at 25°C in a thermostatted cuvette holder. Trastuzumab samples were stained with ANS: 1.3 ml of the ANS stock solution was added to 100 ml of the protein solution. The fluorescence of 1-anilinonaphthalene-8-sulfonic acid (ANS) was monitored between 375 and 650 nm, with an excitation wavelength of 367 nm. The spectra were recorded with a 0.1 second integration time per 1 nm increment. The excitation and emission slits were both set to 1 mm. Steady-state anisotropy measurements were performed using prism polarizers and the anisotropy (A) was calculated from the equation:
A = (I0,0 − G × I0,90) / (I0,0 + 2G × I0,90)
The ANS anisotropy value was calculated from fluorescence spectra between 492 and 512 nm, using an excitation wavelength of 367 nm, with four seconds integration time per nm increment. The excitation and emission slits were set to 2 and 3 mm, respectively.
Fluorescence lifetime measurements.
Fluorescence lifetimes were measured using time-correlated single-photon counting (TCSPC) on an IBH 5000U fluorescence lifetime spectrophotometer (Glasgow, United Kingdom), fitted with a 371 nm NanoLED excitation source and a monochromator at the emission side. Emission wavelength was determined by steady-state fluorescence spectroscopy. Data analysis was performed using the DAS6 software (IBH, Glasgow, United Kingdom). After reconvolution of the intensity decay with the instrument response function, the calculated data were analysed by linear and non-linear least-squares modeling. The average lifetimes were calculated using the equation: where τ is the fluorescence decay time and a the normalized pre-exponential factor.Citation18
Fluorescence microscopy.
Trastuzumab samples were stained with Nile Red: 1 µl of the 100 µM Nile Red stock solution was added to 100 µl of the protein solution. Immediately after staining, trastuzumab samples were placed on Kova Glasstic slides (Hycor, Garden Grove, USA) and observed by fluorescence microscopy. The observations were performed on an Axiovert 200 microscope (Zeiss, Göttingen, Germany) equipped with a mercury discharge lamp. To visualize Nile Red fluorescence, a Zeiss filter cube no15 was used (EX BP 546/12, BS FT 580, EM LP 590). The images were acquired with a cooled Retiga 1300C colour CCD camera (QImaging, Burnaby, Canada) and processed with the Openlab version 3.1.7 software (Improvision, Coventry, UK). The observations were performed using 10×, 20× and 40× A-Plan LD objectives (Zeiss, Göttingen, Germany). Exposure time was 2 seconds. Brightness and contrast were adjusted similarly on all photomicrographs. n indicates the number of particles counted.
Electron microscopy.
A 5 µl aliquot of the sample was incubated for 45 seconds on a freshly glow-discharged copper grid (mesh 200) covered with a parlodium/carbon film. The grid was sequentially washed in three drops of deionized water and stained for 45 seconds with 2% (w/v) freshly diluted and filtered uranyl acetate. The unbound stain was removed from the specimens. The grid was air-dried and analyzed using a Hitachi 7000 (Tokyo, Japan) transmission electron microscope.
Abbreviations
| ANS | = | 1-anilinonaphthalene-8-sulfonic acid |
| EMEA | = | european medicines agency |
| FDA | = | food and drug administration |
| FFF | = | asymmetrical flow field-flow fractionation |
| IgG | = | immunoglobulin G |
| TEM | = | transmission electron microscopy |
Figures and Tables
Figure 1 Trastuzumab diluted in 0.9% NaCl (A) and in 5% dextrose (B) analysed by FFF. Trastuzumab concentration was 1.28 mg/ml and the analysis was performed with 0.9% NaCl as the running buffer (carrier liquid). Separation started at 5 minutes elution time and stopped at 14 minutes elution time. Molecular weights (thick lines, left scale) are superimposed on the UV signals (thin lines, right scale). The monomer peak at 11.5 minutes shows a molecular weight of 160 kDa. The inset provides a magnified view of the chromatograms, showing a second peak at 13 minutes that can be interpreted as small aggregates. With the method used, no differences can be seen between trastuzumab diluted in NaCl and diluted in dextrose.
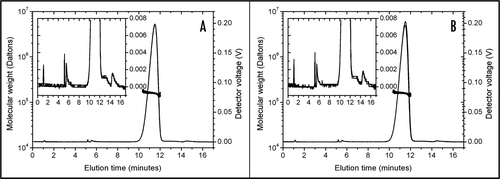
Figure 2 FFF analysis of trastuzumab diluted in 0.9% NaCl (A and C) and in 5% dextrose (B and D). Molecular weights (dots, left scale) are superimposed on the UV signals (lines, right scale). Each colour indicates a separate injection. No focussing or cross-flow was applied. After 1 minute elution time, trastuzumab solutions (1.28 mg/ml) were injected in the channel flow. (A) Trastuzumab diluted in 0.9% NaCl and analysed with 0.9% NaCl as the running buffer. Between 2.5 and 3.5 minutes, only the monomeric protein could be seen. (B) Trastuzumab diluted in 5% dextrose and analysed with 5% dextrose as the running buffer. Recovery was low and variable as shown by the UV signal: part of the sample was adsorbed to the FFF system. Baselines did not come back to zero. Molecular weight determination indicated the presence of large species. (C) Trastuzumab diluted in 0.9% NaCl and analysed with 5% dextrose as the running buffer. Upon contact with 5% dextrose in the FFF channel, trastuzumab immediately aggregated, as indicated by the higher molecular weight, the low and variable recovery and the baselines not coming back to zero. (D) Trastuzumab diluted in 5% dextrose and analysed with 0.9% NaCl as the running buffer. Trastuzumab aggregates (as seen in (C)) were partially disrupted during analysis in 0.9% NaCl. Only the increased molecular weight (between 2.5 and 3.5 minutes) indicated the presence of aggregates.
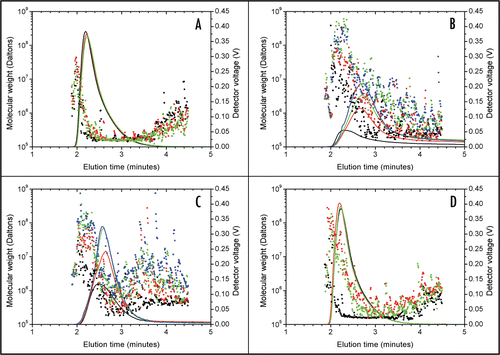
Figure 3 Rinsing of the FFF channel after several injections of trastuzumab in 5% dextrose. Molecular weights (dots, left scale) are superimposed on the UV signal (line, right scale). A thorough rinsing of the channel detached high molecular weight trastuzumab aggregates that were adsorbed on the FFF system.
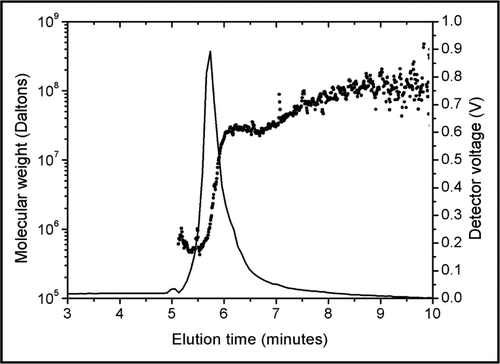
Figure 4 Steady-state fluorescence emission spectra of 50 µM ANS in the presence of NaCl-diluted trastuzumab (solid line) and dextrose-diluted trastuzumab (dotted line). When trastuzumab was diluted in 5% dextrose, ANS exhibited a higher fluorescence as well as a shift to smaller wavelengths, indicative of a stronger binding of ANS to dextrose-diluted trastuzumab than to NaCl-diluted trastuzumab.
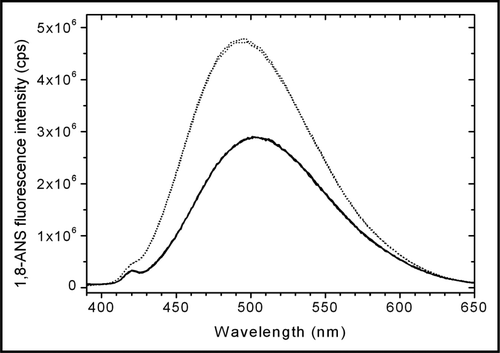
Figure 5 Steady-state fluorescence anisotropy spectra of 50 µM ANS in the presence of NaCl-diluted trastuzumab (solid line) and dextrose-diluted trastuzumab (dotted line). The higher anisotropy of ANS in dextrose-diluted trastuzumab is indicative of a lower mobility of ANS, consecutive to a stronger binding to trastuzumab.
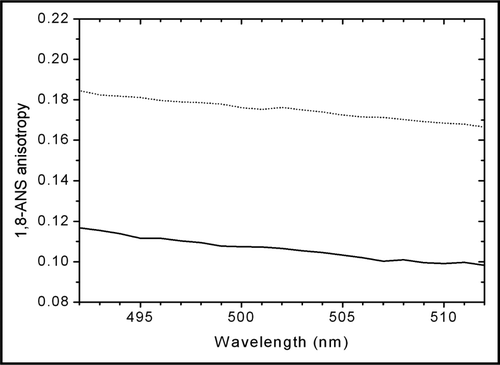
Figure 6 Fluorescence photomicrographs of trastuzumab diluted in 0.9% NaCl (A) and in 5% dextrose (B). Aggregates are designated with arrows. (A) In 0.9% NaCl, trastuzumab aggregates were small (<10 µm). (B) In 5% dextrose, trastuzumab aggregates were larger (most aggregates >10 µm) and present in higher numbers.
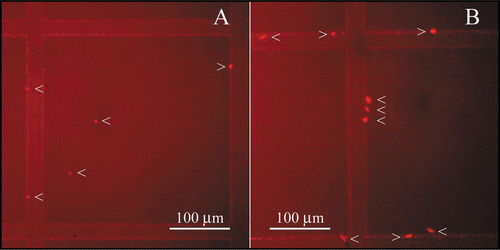
Figure 7 Transmission electron microscopy images of trastuzumab solutions (1.28 mg/ml) in 0.9% NaCl; low magnification, (A); high magnification, (C) and in 5% dextrose, low magnification, (B); high magnification, (D) Aggregates were only present in 5% dextrose and appeared as black spots (B and D).
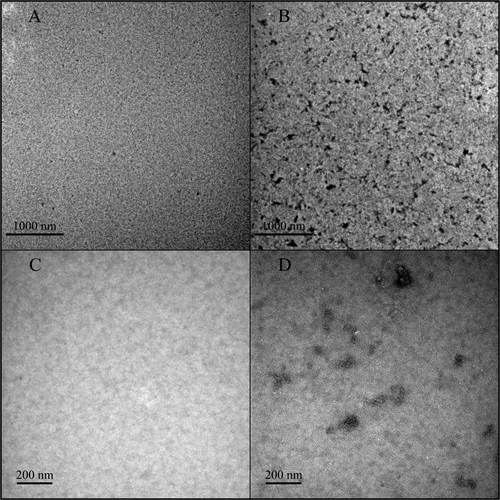
Figure 8 Transmission electron microscopy image of a trastuzumab aggregate formed in 5% dextrose. The aggregate appears amorphous, without fibrillar structure.
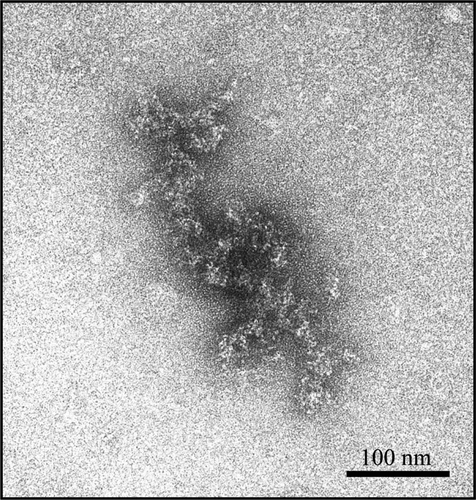
Figure 9 Transmission electron microscopy images of 0.9% NaCl (A) and 5% dextrose (B) solutions, without trastuzumab. No particles are visible.
Table 1 Field-flow fractionation methods used for the characterization of trastuzumab samples
Table 2 ANS fluorescence lifetime parameters for trastuzumab diluted in: (A) 0.9% NaCl and (B) 5% dextrose
Table 3 Comparative table of the analytical techniques presented in this paper
References
- Volkin DB, Mach H, Middaugh CR. Shirley BA. Degradative covalent reactions important to protein stability. Methods in Molecular Biology, Vol. 40: Protein Stability and Folding: Theory and Practice 1995; Totowa, NJ Humana Press Inc 35 - 63
- Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res 2003; 20:1325 - 1336
- Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm 2000; 203:1 - 60
- Chi EY, Weickmann J, Carpenter JF, Manning MC, Randolph TW. Heterogeneous nucleation-controlled particulate formation of recombinant human platelet-activating factor acetylhydrolase in pharmaceutical formulation. J Pharm Sci 2005; 94:256 - 274
- Webb SD, Cleland JL, Carpenter JF, Randolph TW. A new mechanism for decreasing aggregation of recombinant human interferon-gamma by a surfactant: slowed dissolution of lyophilized formulations in a solution containing 0.03% polysorbate 20. J Pharm Sci 2002; 91:543 - 558
- Carpenter JF, Kendrick BS, Chang BS, Manning MC, Randolph TW. Inhibition of stress-induced aggregation of protein therapeutics. Methods Enzymol 1999; 309:236 - 255
- Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm 2005; 289:1 - 30
- Trastuzumab Product Approval Information—Licensing Action 2008 http://www.fda.gov/cder/biologics/products/trasgen092598.htm
- Cudd A, Arvinte T, Das RE, Chinni C, MacIntyre I. Enhanced potency of human calcitonin when fibrillation is avoided. J Pharm Sci 1995; 84:717 - 719
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002; 416:507 - 511
- Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W. Structure-immunogenicity relationships of therapeutic proteins. Pharm Res 2004; 21:897 - 903
- Walsh G. Biopharmaceuticals: recent approvals and likely directions. Trends Biotechnol 2005; 23:553 - 558
- Lawrence S. The biotech drug market. Nat Biotechnol 2004; 22:1496
- European Public Assessment Report for authorised medicinal products for human use—Herceptin 2008 http://www.emea.europa.eu/humandocs/Humans/EPAR/herceptin/herceptin.htm
- Demeester J, De Smedt SS, Sanders NN, Haustraete J. Jiskoot W, Crommelin DJ. Light scattering. Methods for structural analysis of protein pharmaceuticals 2005; Arlington, VA AAPS Press 245 - 275
- Tessier PM, Lenhoff AM. Measurements of protein self-association as a guide to crystallization. Curr Opin Biotechnol 2003; 14:512 - 516
- Bajaj H, Sharma VK, Kalonia DS. Determination of second virial coefficient of proteins using a dual-detector cell for simultaneous measurement of scattered light intensity and concentration in SEC-HPLC. Biophys J 2004; 87:4048 - 4055
- Lakowicz JR. Time-domain lifetime measurements. Principles of Fluorescence Spectroscopy 1999; Second edition New York Kluwer Academic/Plenum Publishers 95 - 140
- Ingle JD, Crouch SR. Ingle JD, Crouch SR. Molecular scattering methods. Spectrochemical analysis 1988; Upper Saddle River Prentice-Hall 494 - 524
- Gabellieri E, Strambini GB. ANS fluorescence detects widespread perturbations of protein tertiary structure in ice. Biophys J 2006; 90:3239 - 3245
- Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res 2008; 25:1487 - 1499
- Demeule B, Gurny R, Arvinte T. Detection and characterization of protein aggregates by fluorescence microscopy. Int J Pharm 2007; 329:37 - 45
- Arvinte T. Jiskoot W, Crommelin DJ. Analytical methods for protein formulations. Methods for structural analysis of protein pharmaceuticals 2005; Arlington, VA AAPS Press 661 - 666
- Gabrielson JP, Brader ML, Pekar AH, Mathis KB, Winter G, Carpenter JF, et al. Quantitation of aggregate levels in a recombinant humanized monoclonal antibody formulation by size-exclusion chromatography, asymmetrical flow field flow fractionation, and sedimentation velocity. J Pharm Sci 2007; 96:268 - 279
- Liu J, Andya JD, Shire SJ. A critical review of analytical ultracentrifugation and field flow fractionation methods for measuring protein aggregation. AAPS J 2006; 8:580 - 589