Abstract
Monoclonal antibody (mAb) deals notable in size have made headlines recently. While deals with over US$ 1 bio in milestones were highly unusual in the past, even preclinical agreements, including some with very attractive co-promotion or profit-sharing clauses for the licensor, now reach this mark. This article presents an analysis of the structure of high-value mAb deals and the impact of increasingly sophisticated terms. Strategies of licensees and the impact of strategy on a deal’s size and structure are also examined.
Recent Deals and Deal Value
Although numerous mAb deals of more than US$ 1 bio value have been reported, usually very limited information is available about the deal structures and the precise motives of the partners. Nevertheless, we can closely examine some deals, analyze their structures and determine how they might fit hypothetical strategies of the participating companies. The two cases selected for analysis were the Regeneron Sanofi-Aventis deal and the agreement between PDL BioPharma and Bristol-Myers Squibb.
Five recent mAb deals have exceeded US$ 1 bio in value (). The deal value is usually the sum of all mentioned milestones of the agreement. These agreements often include many indications, and even back-up programs. The scenario where all milestones will be paid out is highly improbable, but not impossible. For example, Genmab is likely to receive a number of the agreed upon milestones because the partnered project, HuMax-CD20 (ofatumumab), is currently being tested in at least nine indications.
So, the reported deal value is likely an exaggeration of the actual value of the deal and provides only limited insight into the deal. The milestones are neither risk-adjusted, i.e., reduced by the likelihood that they actually occur, nor are they discounted. Sales milestones, in the case of the Regeneron Sanofi-Aventis deal of $250 Mio, will only be triggered in ten years or more, and only if the project reaches the market. Using a discount rate of 12% and standard success rates for a new investigational humanized mAb drug (Reichert)Citation3 of 32% (success rates for fully human mAb are not available) this means that the $250 Mio only represent $25 Mio in actual value. Furthermore, some milestones might never be triggered because they relate to an indication that will never be developed or to sales targets that are too high. The reported $400 Mio milestones in the MorphoSys-Novartis deal are an exception because they are risk-adjusted.
Another misleading feature of the notion of “deal value” is that it does not include royalties. Royalties can make up more than half of the complete actual deal value if calculated with standard risk-adjusting valuation methods, but this depends on whether the deal is front or back-loaded. The same is true for co-development and profit sharing agreements. These clauses, which can be very valuable for the licensor, are also not considered in the deal value. We will demonstrate that these clauses can be of much greater importance than the sum of potential milestones.
Structure of Deals: Regeneron and Sanofi-Aventis
The Regeneron Sanofi-Aventis deal as displayed in Box 1 provides an excellent illustration of the different components of the modern mAb mega-deal. The deal exhibits most clauses that have become increasingly popular:
Equity investments
Inclusion of several compounds (technology deals)
Earlier development undertaken by biotech linked to R&D funding
Later redemption of funding
Inclusion of sales milestones
Co-promotion and profit sharing
All of these terms suggest that biotechnology companies have a strong position when negotiating deals with big pharmaceutical firms. However, the analysis is strongly biased when we only look at the high-value deals.
Equity investments.
Many deals include an equity investment of the pharma partner in the biotech company. The infused cash liberates the biotech company from the time-consuming, and attention diverting, process of fund raising rounds. The equity investment, which is often substantial, also ties the two companies closer together. An equity investment does not increase the value of a deal; it is an exchange of shares against cash. Nevertheless, the pharmaceutical industry often perceives equity as another form of upfront payment and writes it off.
Technology deals.
Regeneron has closed a technology deal that includes projects generated over several years (similar to OncoMed and MorphoSys). The terms depend on sales performance, i.e., sales milestones and profit sharing, relating to aggregated sales stemming from all products included in the deal. Usually, these terms depend only on project-by-project sales. Such terms are favourable to the biotech company because it is easier to reach milestones if these sales stem from a variety of products rather than from just one. In general, tiered royalties, tiered profit sharing and sales milestones are reached faster if they depend on portfolio performance rather than project performance. Valuation of deals with tiered royalties (or tiered profit share schemes) and sales milestones on a portfolio level (several projects or also several indications) are quite difficult because we cannot simply value each project and add up all project values. With one project alone, some sales milestones might never be triggered or some royalty tiers might not be reached; we therefore also have to consider scenarios where projects jointly reach the market.
Co-development and profit sharing.
Regeneron and Sanofi-Aventis have agreed to co-develop the program and to share profits. The PDL BioPharma and Bristol-Myers Squibb deal also has a similar structure. Sanofi-Aventis will pay all development costs upfront. If marketing approval is achieved, then Regeneron will pay its share of the development costs from the first profits. This repayment schedule is very favourable to Regeneron's deal value because the development costs will be paid later, and only in the case of marketing approval. We can assume that Sanofi-Aventis cushioned this effect somewhat with a formula for repayments that was mentioned, but not shown. However, the risk reducing effect for Regeneron remains—it only has to pay for the trials once it is clear that they are successful. Profits will be shared 50%–50% in the US, and according to a sliding scale outside of the US. Sanofi-Aventis receives 65% of profits for ex-US sales up to $750 Mio and 55% above. This can be seen as compensation for the unbalanced risk position with respect to clinical trial costs.
Deal mechanism.
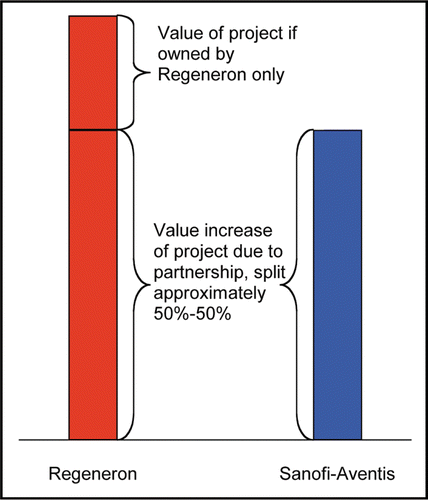
The mechanism of the deal is illustrated by examination of the various deal components (). Upfront or milestone payments and R&D funding are exactly the opposite for Sanofi-Aventis and Regeneron (assuming that both use the same discount and success rates). These terms basically correspond to the price Sanofi-Aventis has to pay in order to participate in the project; in the beginning Regeneron owns the project and Sanofi-Aventis must compensate Regeneron for the share it claims in future profits. Furthermore, the clinical costs differ as discussed, and this is compensated by a higher profit share outside the US. Sanofi-Aventis' value increases relative to Regeneron's value if ex-US sales increase. The tiered profit sharing for ex-US sales suggests that the deal is probably intended to represent a 50%–50% partnership between the two companies, once Sanofi-Aventis compensated Regeneron's value creation up to deal closing with upfront, R&D funding, and milestones. The difference between Regeneron's and Sanofi-Aventis' value can be interpreted as the initial value of the project prior to the agreement, and the remainder of the value (i.e., twice Sanofi-Aventis' value) stems from the partnership ().
Regeneron and Sanofi-Aventis, November 29, 2007
$312 Mio equity investment. Sanofi-Aventis increases its stake to approximately 19%.
$85 million upfront payment.
Sanofi-Aventis funds up to $475 million of research over the next five years.
Sanofi-Aventis will have an option to extend the research agreement for up to an additional three years.
Sanofi-aventis will have the exclusive option to co-develop with Regeneron each drug candidate in the collaboration portfolio.
Development costs will be shared between the two companies, with Sanofi-Aventis funding drug candidate development costs up front (phase I and II 100% by Sanofi-Aventis, Phase III 80%) and Regeneron reimbursing half of the development costs from its share of future profits.
Sanofi-Aventis will take the lead in commercialization activities and will consolidate the sales.
Regeneron will have the right to co-promote any and all collaboration products worldwide.
In the United States, profits will be shared equally.
Outside the United States, profits will be split on a pre-determined sliding scale with Sanofi-Aventis' share ranging from 65%to 55%.
Regeneron will be entitled to receive up to a total of $250 million of sales milestone payments when the collaboration achieves certain aggregate annual ex-U.S. sales levels, starting at $1 billion.
Regeneron responsible for manufacture of clinical supply.
If Sanofi-Aventis does not exercise option, Regeneron retains global rights and Sanofi-Aventis entitled to royalties.
Standstill agreement: Sanofi-aventis restricted from increasing ownership of Regeneron common stock to more than specified percentages that increase over time. Maximum of 30% ownership beginning four years after the stock purchase.
Source: Press release onwww.regeneron.com, and presentation by Sanofi-Avantis
The deal rationale illustrated in is only valid as long as we apply the same discount rate to Regeneron and Sanofi-Aventis. In reality, Sanofi-Aventis will probably experience a lower cost of capital. This implies that Sanofi-Aventis' value will increase and Regeneron's value will decrease. So, Sanofi-Aventis' actual deal value might be greater than Regeneron's, although Regeneron started ahead with the initial project value. Nevertheless, the 50%-50% partnership might remain a viable deal rationale given the disclosed terms.
Deal Analysis: PDL BioPharma and Bristol-Myers Squibb
The PDL BioPharma and Bristol-Myers Squibb deal was also dissected to determine the benefits to each party. This deal was similar to the Regeneron-SanofiAventis deal, but was less complex because there was no equity investment and no R&D funding, the deal related to only two products, and it did not include profit sharing in non-US countries (cf. Box 2). For simplicity, we included only one candidate, elotuzumab, in the analysis of the deal (PDL241 will not be considered).
The goal was to analyze the deal so that the analysis could be used for deals that refer to other compounds. The typical parameters of interest are value share, internal rate of return (IRR) for licensee or royalty rates. Therefore, we need to estimate several parameters that are linked to the project and others that are linked to the license contract. For the estimations of project parameters, we relied mainly on published values ().
We assumed operating expenses of 35% once the project is on the market, which is in line with reported financials from companies that market mAb therapeutic products.
The companies have communicated only fragments of the license terms. The upfront payment of $30 Mio and the total of milestones of $480 Mio and $200 Mio of sales milestones were disclosed. Furthermore, we know that the development costs were to be split 80%-20% between Bristol-Myers Squibb and PDL BioPharma. The exact distribution of the milestones over phases and indications, and also the royalty rates for ex-EU sales remain undisclosed. Thus, milestones and royalties have to be estimated. For this, metrics previously developed (Bogdan, Villiger)Citation1 by analysing other license contracts were used. Milestones are usually up-stepped, i.e., they increase with ongoing progress of the project. Analysis of discovery and preclinical datasets suggest increasing milestones (). The weights indicate that milestones belonging to that phase are a multiple of the upfront payment. The milestones can be split over several indications, and approval milestones include sales milestones that follow later.
In this case, we have $30 Mio upfront payment and a total deal value of $710 Mio (= 30 + 480 + 200). These numbers do not exactly fit into the datasets, because $710 Mio is only 23.7 times $30 Mio. But we remember that the upfront payment also included an option payment for a second compound, PDL241. If we assume this option payment to be about $7.5 Mio the upfront payment for elotuzumab amounts to $22.5 Mio, which is the 31.5th part of $710 Mio. With this in mind, the milestones could be as in .
We can also expect that the milestones are distributed over several indications, with the first being the most important one. Typically such deals include milestones for several cancer indications and also one non-cancer indication. The actual milestones could be as in .
The exact trigger points of sales milestones and royalties are unknown. We can assume that royalties will range from 15%–20%, and might perhaps be higher. The sales milestones amount suggests that the peak sales expectation is rather elevated. We can assume a first milestone of $100 Mio after having sold $1,000 Mio ex-US, and a second milestone of $100 Mio after having sold another $1,000 Mio ex-US.
Given the 50%-50% profit sharing and the high milestone terms, we expect PDL BioPharma to keep a large portion of the value. Running a valuation with several indications at a 12% cost of capital and a preliminary royalty structure of 15% up to $500 Mio and 20% above, we get the results shown in .
The valuation results indicate that, under these assumptions, PDL BioPharma negotiated an excellent deal. Usually, the licensee is expected to get about 75% of a phase I deal. In this case, Bristol-Myers Squibb reaches a 50% share if elotuzumab reaches $2.5 bio in sales.
Although the calculations are based on various estimations, they still provide insight into how these deals create value for both parties. This analysis suggests several conclusions. First, it is entirely possible that the deal is more back-loaded than forecasted in . The phase II milestones for 2nd and 3rd indication seem high, but the effect of back-shifting the milestones will not significantly change the value share. Second, the assumed royalty rates of 15% and 20% might be too high. We think that this order of magnitude is justified, given the milestone amounts. Third, the milestones might be distributed over more than three indications, resulting in fewer milestones per indication. While these three points all have a small affect favouring Bristol-Myers Squibb, we haven't accounted for indication-specific success rates. Success rates are usually assessed per candidate, i.e., if one indication moves to another phase then the project is counted as success, even if many other indications fail. Therefore, a single indication has a lower probability of success than suggested by published success rates. This would shift the value share against Bristol-Myers Squibb. A fourth explanation for why the outlined analysis might fall short in explaining Bristol-Myers Squibb's potential is the reduced threat of generic competition. The commercial life cycle of the mAb product might last much longer than only until patent expiry (as assumed in the analysis) because biosimilar mAbs have yet to be approved in either the US or EU [Schneider, Kalinke].Citation6 Addition of five years to the life cycle for the middle scenario leads to a value split of $227 Mio—$283 Mio (44.5%–55.5%). As a fifth point, a more optimistic assessment of success must be considered. This is contrary to the possibility of lower indication success rates, but examination of the potential value development and the corresponding upside ( and ) provides further understanding of why pharmaceutical partners are ready to pay such high terms.
PDL BioPharma and Bristol-Myers Squibb, August 19,2008
Bristol-Myers Squibb Company and PDL BioPharma, Inc. enter an agreement for the global development and commercialization of PDL BioPharma's anti-CS1 antibody, elotuzumab, currently in Phase I development for multiple myeloma.
Upfront cash payment of $30 million for the development and marketing rights to elotuzumab and for an option to expand the collaboration to include PDL241.
Additional payments of up to $480 million based on pre-defined development and regulatory milestones.
Up to $200 million based on pre-defined sales-based milestones.
The companies will share development costs, with Bristol-Myers Squibb providing 80% of the funding and PDL BioPharma providing 20%.
Bristol-Myers Squibb will lead global development activities.
PDL BioPharma will complete the ongoing Phase I program and provide support for Phase II studies.
The companies would share profits on sales of elozutumab in the U.S. PDL BioPharma would receive royalties on net sales outside the U.S.
If Bristol-Myers Squibb exercises its option to expand the collaboration to include PDL241, PDL BioPharma would receive an additional cash payment of $15 million and could receive additional payments of up to $230 million based on predefined development and regulatory milestones and up to $200 million based on pre-defined sales-based milestones. The same division of development costs and profit sharing that apply to elotuzumab would apply to PDL241.
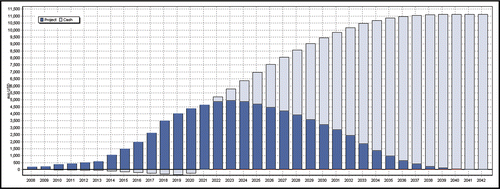
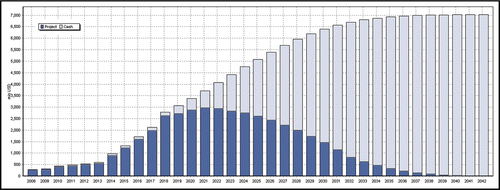
Although Bristol-Myers Squibb () starts at a lower value at deal closure than PDL BioPharma (), the company can generate more cash from the project in the case of success ($11 bio vs. $7 bio). Bristol-Myers Squibb must invest up to $400 Mio by 2019, but will then cash in more revenues than PDL BioPharma due to its larger stake in the non-US market. On the other hand, PDL BioPharma securitises a part of the project value relatively early in the development through milestone payments. As a sixth and last point, the concept of value share becomes less important when the project itself has a huge potential. Calculated at 12%, the deal represents about $200 Mio to Bristol-Myers Squibb (taxes have not been considered), which is a lot for a phase I project. The company could increase its pipeline value by the same amount just by signing the deal.
Strategic Considerations
In addition to the technical analysis of how to construe such deals, we want to shed light on the underlying strategies of the parties. It is undisputed that the pharma partners are paying higher prices, but there could be various reasons for this. Since most big deals involve mAbs, the high deal terms might be linked to either a much higher expected sales potential for the products, or the fact that these deals are mostly linked to a technology exchange. The biotech partners can, or even must, ask for such high entry payments because they grant access to their technology, thereby giving away part of a large upside potential. Pharmaceutical company partners can act as a barrier to consideration of either the technology or the biotech partner by other companies. The pharma partners might also facilitate a potential later take-over of the biotech partner, since they own already part of a product, and possibly also some equity.
Regeneron included a standstill clause in the agreement with Sanofi-Aventis that prevented Sanofi-Aventis from taking over Regeneron completely. This clause counters the generally prevailing opinion that the pharma companies are going to take over their licensors sooner or later. On the other hand, Regeneron might have wanted to ensure that, in the case of a take-over, their investors are only bought out once the full value potential is evident.
Investors appreciated the deal with Sanofi-Aventis; the share price rose 35% upon announcement. However, PDL BioPharma's deal with Bristol-Myers Squibb only led to a 3% increase. Sometimes a deal is already included in the valuation, or investors don't necessarily see a licensing deal as the better alternative to continuing the development within the company. It has been claimed that license deals could also be value destructive, as they make a take-over by a company other than the licensee much less likely (Longman).Citation7 The approval effect of a license deal with a big pharmaceutical company player has recently lost importance, and investors seem to care more about the lost potential upside. However, this is more important for private companies, where a trade sale represents an important potential exit scenario for investors. A public company has to care less about exit scenarios, as investors can simply sell their stakes. In any case, a biotechnology company must compare the term sheet it has on the table with the alternatives, i.e., moving the product forward with its own means and diluting its investors. Closure of a license deal by a public company might also be an indication that the company feels undervalued by the public markets.
It is difficult to judge exactly how investors react to these deals in the mid-term because several effects overlay each other. An immediate share price reaction indicates investors approved the deal, but we have to wait until a few years have passed before we can judge what will come of these recent mega-deals. Only then we can see whether biotech, pharma, or potentially both, were the winners.
Conclusion
Our analysis of two large mAb deals has shown that biotechnology companies are able to negotiate much better deal terms, even for early stage projects, than usually assumed in the industry—the exhibited value shares of more than 50% for the licensor exceeded the standard 25% for IND deals by far. The projects obviously exhibit a huge potential. MAbs exhibit a better investment profile compared to other drug classes because several commercialised mAbs have blockbuster status (annual global sales exceeding US$1billion), and mAbs cannot be easily replaced by generics. As a sign of biotech's improved bargaining position the pharmaceutical partners have to concede profit sharing models to the presumed junior partners. Nevertheless, our analysis has shown that big pharma still has leverage. Even if profit sharing clauses suggest an approximate 50%–50% value share, as in the two analyzed deals, pharmaceutical companies still profit from a much lower cost of capital. Therefore, pharmaceutical partners probably still receive a larger piece of the pie than biotech. While the US$1 bio milestone figures are certainly notable, they flatter biotech. The biotechnology company's value is much lower due to risk adjustments and high costs of capital, even though the so-called deal values do not include royalties and profit share clauses. Venture capital investors have recently been resistant to premature license deals as these impede very attractive trade as exits. Public companies face the same situation, but they might close a mega-deal to signal that they feel undervalued by the market.
Figures and Tables
Figure 1 Mechanism of deal terms in the Regeneron Sanofi-Aventis contract. The final value (columns on the right) is built by many components. These components do not always impact the value in the same way for licensor and licensee..
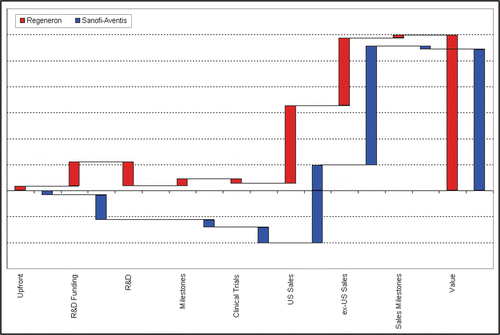
Table 1 Recent mega-deals with monoclonal antibodies
Table 2 R&D valuation assumptions
Table 3 Milestones usually keep increasing with progress of project
Table 4 Possible milestones for elotuzumab
Table 5 Possible milestone structure for elotuzumab
Table 6 Results for various sales scenarios, calculated with ri:val
References
- Bogdan B, Villiger R. Valuation in Life Sciences. A Practical Guide 2008; Second Edition Springer Verlag 171 - 173
- DiMasi J, Grabowski H. The Cost of Biopharmaceutical R&D: Is Biotech Different?. Manage Decis Econ 2007; 28:469 - 479
- Reichert J. Success and failure: Clinical trials of protein therapeutics 2005; Conference on plant-made pharmaceuticals
- Reichert J, Rosensweig C, Faden L, Dewitz M. Monoclonal antibody successes in the clinic. Nat Biotechnol 2005; 23:1073 - 1078
- Reichert J. Biopharmaceutical Approvals in the US Increase. RAJ Pharma 2004;
- Schneider, Kalinke. Toward biosimilar monoclonal antibodies. Nat Biotechnol 2008; 26:985 - 990
- Longman R. Why Investor's Don't Like Biotech Alliances?. IN VIVO Blog 2008; March