Abstract
Although immunoglobulin (Ig) A is commonly recognized as the most prevalent antibody subclass at mucosal sites with an important role in mucosal defense, its potential as a therapeutic monoclonal antibody is less well known. However, IgA has multifaceted anti-, non-, and pro-inflammatory functions that can be exploited for different immunotherapeutical strategies, which will be the focus of this review.
Introduction
In the human body, more immunoglobulin (Ig) A is produced per day (66 mgkg−1day−1) than all other antibody isotypes combined.Citation1 Moreover, in addition to being the most prominent antibody class at mucosal sites, IgA is the second prevalent antibody in the circulation. Although notable breakthroughs in understanding the role of IgA and IgA receptors have been achieved in the last decade, IgA is generally, but erroneously, still considered to be primarily a non-inflammatory antibody that helps to maintain homeostasis in the mucosa.Citation2 However, through different expression forms and interaction with several distinct receptors, IgA can passively and actively inhibit or initiate inflammatory responses. The prototypic myeloid IgA Fc receptor FcαRI (CD89) plays a key role in several of these processes.Citation3,Citation4 As such, the role of IgA and FcαRI in immunity and their potential for immunotherapeutic strategies deserve re-evaluation. Because the potential of IgA as an anti-inflammatory agent was recently reviewed in reference Citation5 and Citation6, this review mainly addresses the possible uses of IgA monoclonal antibodies (mAbs) to actively target FcαRI for treatment of infections and cancer.
The Basics of IgA
One fundamental reason why the role of IgA in immunity can be misinterpreted is the dissimilarity between IgA systems in humans compared with the rodents that are commonly used for experimental work.Citation7,Citation8 IgA mostly presents as a monomer in human serum, whereas it is a polymeric molecule in the circulation of most animal species. As such, clearance through the hepatobiliary route is important in mice, but not in humans.Citation9 Moreover, in humans IgA exists as the two closely related subclasses IgA1 and IgA2 [subdivided in IgA2m(1) and IgA2m(2)], which differ over a stretch of 18 amino acids in their hinge region, with the hinge of IgA1 being 13 amino acids longer than that of IgA2 ().Citation1,Citation10,Citation11 Consequently, based on molecular modelling, it is predicted that IgA1 has a broader reach that is beneficial in antigen recognition with distantly spaced antigens, but at the cost of augmented susceptibility for proteolysis. Because IgA2 lacks this extended hinge region, it is less vulnerable to IgA1 bacterial proteases, which may underlie the predominance of IgA2 in mucosal secretions, although it is debatable whether IgA2 is less vulnerable in general.Citation12 Furthermore, post-translational glycosylation varies between IgA isotypes and sub-isotypes. IgA1 has two conserved N-linked glycosylation sites (Asn-263 and Asn-459), whereas IgA2 has either two (IgA2m1) or three (IgA2m2) additional N-linked glycosylation sites. IgA1 also harbours O-linked glycosylation in the hinge region, in contrast to IgA2.Citation13–Citation16 Even though IgA is considered a poor complement activator because it cannot bind C1q, IgA can activate the MBL-lectin complement pathway.Citation17
In humans, IgA is expressed in three different forms. One to three mg/mL serum IgA is present in the circulation as monomer, whereas IgA at mucosal sites is produced as polymeric molecules. X-ray and neutron scattering analyses suggest that IgA adopts average T-shaped structures.Citation18 Most mucosal plasma cells produce dimeric IgA, which incorporates a disulfide-bridge at the C-terminus of a single α-chain of each IgA molecule with a 16 kDa joining J-chain ().Citation9,Citation19,Citation20 Dimeric IgA, containing J-chains bind to the polymeric Ig-receptor (pIgR), which is expressed on the basolateral membrane of epithelial cells, after which it is transported through epithelial cells and released into the lumen as secretory IgA (SIgA).Citation20–Citation22 IgA is secreted via this route into the mucous lining of the gastrointestinal, urogenital and respiratory tracts, as well as into tears, saliva and milk.Citation23 Apical cleavage of the pIgR ensures that a part of this receptor, referred to as secretory component (SC), remains attached to IgA (), which stabilizes IgA and prevents rapid breakdown in the hostile environment of the gut lumen.
IgA Receptors
Another notable difference between human and animal IgA systems is the diversity of IgA receptors between species, which complicates comparisons. The existence of receptors for IgA was initially proposed after binding of IgA1 myeloma protein and SIgA to blood neutrophils was observed.Citation24 Currently, multiple types of cellular IgA receptors that can either bind the Fc tail, carbohydrate side chains or accessory molecules like the J-chain and SC, including pIgR,Citation19,Citation25 Fcα/µ receptors,Citation26 asialoglycoprotein-receptors,Citation27 transferrin receptors (TfR, CD71),Citation28 SC receptors,Citation29 M-cell receptors,Citation30 have been identified in both rodents and humans. The functions of a number of these receptors have not yet been completely elucidated, but do not seem to differ greatly between species, although subtle variations can be observed. The major divergence is the lack of the myeloid FcαRI in mice. Several bacterial IgA proteins that bind to sites in IgA that overlap with the binding site of FcαRI have also been described, e.g., IgA-binding M-like proteins Arp4, Sir22, b-antigen and members of the staphylococcal superantigen-like proteins (SSL) family.Citation31 As such, a main role for FcαRI in immune defence is supported, since bacterial evolution has led to development of molecules that interfere with IgA binding to FcαRI, resulting in an important evasion strategy for pathogens to escape IgA-mediated phagocytosis.
Structure and expression of FcαRI
Although FcαRI is a member of the Fc receptor immunoglobulin superfamily, distinct differences can be observed when it is compared with other Fc receptors. For instance, the FcαRI gene is located on chromosome 19 (at 19q13.4) and lies within the so-called leukocyte receptor cluster (LRC),Citation32,Citation33 whereas other FcR genes, such as FcγRs and FcɛRI genes, map on chromosome 1. The human LRC includes no other Fc receptor genes, but instead encodes killer inhibitory receptors (KIR) and leukocyte Ig-like receptors (LIR). FcαRI exhibits more sequence similarities with these receptors than with other Fc receptors.Citation34
Thus far, FcαRI has been identified in primates, horses, cattle and rats, but not in mice, which is likely attributable to a gene translocation in the LRC locus.Citation35–Citation39 FcαRI expression begins at the promyelocyte stage in differentiation and is restricted to cells of the myeloid lineage, including neutrophils, eosinophils, monocytes and most macrophages (alveolar, tonsilar and splenic, but not macrophages from the small intestine). FcαRI is furthermore expressed on Kupffer cells and on interstitial and monocyte-derived dendritic cells.Citation40–Citation48 Expression has recently been described on human platelets as well, but FcαRI is not observed on mast cells or basophils.Citation49 FcαRI expression is constitutive and independent of its ligand, which is demonstrated in IgA deficient patients who still express FcαRI.Citation50 However, expression levels can be modulated by cytokines (depending on cell type),Citation34 or adaptor protein binding to the intracellular domain of FcαRI,Citation51 which can induce either de novo synthesis or transport from intracellular stores to the cell surface.Citation52,Citation53
FcαRI consists of two extracellular Ig-like domains, a 19 aa transmembrane region and a short (41 aa) cytoplasmic tail. For most functions, association with the common FcRγ chain is necessary (see below).Citation54–Citation58 In addition to the full length FcαRI, several splice variants on the mRNA level have been described, and at least two other isoforms exist in vivo. One isoform (FcαRIa.2) is exclusively expressed on alveolar macrophages and differs by a deletion in the extracellular domain (EC) 2, whereas the transmembrane/intracellular domains are deleted in the other isoform (FcαRb), resulting in a soluble receptor.Citation44,Citation59 Furthermore, several polymorphisms have been described of which the Ser-248/Gly-248 polymorphism—which is located in the intracellular domain—has functional consequences and is associated with systemic lupus erythematosus.Citation60
The FcαRI binding site for IgA is located in the extracellular domain EC1,Citation61–Citation63 which is different compared to FcɛRI and FcγRs, as these FcRs bind their ligands in EC2. Residues Y35 (in the BC loop), R52, R53, L54, K55 (in the D strand), F56, W57, N58 (in the DE loop), Y81, R82, I83, G84, H85 and Y86 (in the FG loop) within EC1 are involved in IgA binding.Citation62,Citation64,Citation65 The two EC domains are oriented at approximately 90° relative to each other. Although a number of conformational changes have been observed within the FcαRI-EC1 domain (in the D-strand, DE and FG loop) after binding to IgA, the orientation of the EC domains does not change significantly. FcαRI is a heavily glycosylated protein harbouring six N-glycosylation sites and several putative O-linked glycosylation sites.Citation41 Deglycosylation of FcαRI N58 increases IgA binding.Citation66 Recently, it was described that the pentraxin C-reactive protein (CRP) can bind to FcαRI, which induces cellular activation. However, the pentraxin-binding site on FcαRI is distinct from that of IgA.Citation67
Crystallographic studies demonstrated that one IgA molecule can simultaneously bind two FcαRI molecules ().Citation61,Citation62 This is in contrast to FcγRIII and FcɛRI, for which a 1:1 stoichiometry with their respective ligands was described, again emphasizing the dissimilarities between FcαRI and other members of the Fc receptor family.Citation68–Citation71,Citation72 Because of partial overlap of the IgA binding site for FcαRI and pIgR, binding of SIgA to FcαRI is (partly) hampered due to steric hindrance of SC, although binding is increased when complement receptor 3 acts as co-receptor.Citation62,Citation63,Citation73 Still, SIgA is a poorer opsonin compared with dimeric or monomeric IgA, and is therefore mostly considered as a non-inflammatory variant of IgA. In contrast, dimeric IgA can potently trigger inflammatory functions through cross-linking of FcαRI,Citation74,Citation75 and can thus be considered a pro-inflammatory antibody. However, monomeric IgA probably represents the most interesting form of IgA, as either anti-inflammatory or potent pro-inflammatory responses are induced, depending on the mode of interaction with FcαRI ().
IgA mediated FcαRI signaling and cellular functioning.
Monomeric serum IgA binds with moderate affinity to FcαRI (Ka = ∼106 M−1) in the boundaries of Cα2 and Cα3, whereas IgA immune complexes bind avidly.Citation61,Citation62,Citation64,Citation65,Citation76 Residues within IgA involved in FcαRI binding are L256, L257, L258 in α-helix of AB loop of Cα2 and within Cα3: E348 (A-strand), R382, L384 (C-strand), S387, E389 (CC′ loop), M433, H436 (F-strand), E437, A438, L439, P440, L441, A442 (FG loop), F443, T444 and Q445 (G strand).Citation62,Citation77 IgA Fc glycosylation is not critical for binding to FcαRI.Citation14,Citation78 Immune complexes with optimal binding contain five to six molecules of IgA per complex.Citation79 On monocytes and eosinophils, inside-out signaling is involved in binding, which entails that stimulation of these cells with cytokines rapidly modulates binding capacity in response to intracellular signals, without affecting receptor expression levels.Citation80–Citation83 Thus, in a resting state FcαRI exhibits low capacity to interact with IgA-immune complexes, but ligand binding capacity increases profoundly after stimulation with cytokines like granulocyte/macrophage-colony stimulating factor, and interleukin (IL) 4 or 5, but surface receptor expression is not augmented. This process critically depends on the intracellular domain of FcαRI and the presence of an intact cytoskeleton, but does not require FcRγ chain. It further involves activation of phosphoinositide-3-kinase (PI-3K), intracellular phosphorylation of Serine 263 of FcαRI and binding of the serine/threonine phosphatase protein PP2A. Inside-out signaling is likely not influenced by the Ser-248/Gly-248 polymorphism.
Binding of IgA-immune complexes (containing either monomeric IgA or dimeric IgA) induces pro-inflammatory responses, which requires association of FcαRI with the FcRγ chain subunit.Citation54,Citation55,Citation84 FcαRI contains a positively charged amino acid on position 209 that associates with an opposite negatively charged amino acid of the FcRγ chain, which is necessary, but not sufficient, for tethering FcαRI to FcRγ chain. For this, a more extensive interface between both transmembrane regions is required.Citation54–Citation58 FcαRI can also be expressed in the absence of FcR γ chains, which has been described for transfected cell lines, and selective monocyte and neutrophil populations (). Functionality of FcαRI “γ-less” receptors is limited to ligand binding (inside-out signaling) and receptor internalization,Citation85–Citation87 although Gly248-FcαRI can trigger IL-6 production in the absence of FcRγ chain.Citation88
After cross-linking of FcαRI by IgA-immune complexes, Src kinase Lyn phosphorylates the tyrosines within the associated FcRγ chain immunoreceptor tyrosine-based activation motifs (ITAM). The phosphorylated tyrosines then serve as “docking” sites for recruitment of other tyrosine kinases, including Syk, Blk, Btk, PI-3K and PLC-γ. FcαRI can also associate with members of the Ras/Raf1 pathway, e.g., Grb2, Shc and SHIP.Citation34 Dissimilarities in signaling pathways are induced at inflammatory sites by diverse stimuli and vary between different cell types, but result in pro-inflammatory cellular processes like phagocytosis, antigen presentation, antibody-dependent cellular cytotoxicity (ADCC), superoxide production or cytokine release.
Recently, a new type of intricacy was elucidated for IgA-mediated FcαRI signaling, as it was demonstrated that non-targeted monomeric serum IgA transduces inhibitory signals through FcαRI, which diminishes signaling through other activating Fc receptors.Citation89,Citation90 The ability of monomeric serum IgA to downregulate IgG-mediated phagocytosis, chemotaxis, bacterial activity, oxidative burst activity and cytokine release has been described for some time, but the mechanisms were poorly understood.Citation91–Citation97 Pasquier, et al. unravelled the underlying molecular mechanism by showing that SHP-1 is recruited to FcαRI-associated FcRγ chain, which blocks activating signals via Syk, induced by other Fc receptors.Citation89 This inhibitory capacity through FcRγ chain ITAM is referred to as ITAMi.Citation5 Thus, both IgA-induced activating and inhibitory signals depend on FcαRI-FcR γ chain ITAM, but differ in the recruitment of tyrosine kinases versus tyrosine phosphatases, respectively (). As such, it has been proposed that cross-linking of FcαRI during infection with IgA-opsonized pathogens results in pro-inflammatory responses, whereas naturally occurring serum IgA (not complexed with an antigen) induces inhibitory signals through FcαRI to dampen excessive immune responses (initiated by other Ig-immune complexes). Therapeutic strategies to target ITAMi with monomeric IgA have recently been reviewed by Monteiro, et al.Citation5,Citation6
IgA mAbs and Targeted Therapy
Research to investigate the potential of IgA mAbs for immunotherapeutical approaches is mostly based on in vitro experiments, as in vivo studies have been hampered by the lack of adequate mouse models. Because mice do not express FcαRI, experiments need to be performed in FcαRI transgenic mice.Citation98 Furthermore, as traditional hybridoma technology has yielded murine antibodies, it has been difficult to generate suitable IgA mAbs because murine IgA binds poorly to human FcαRI. Alternatively, IgA mAbs have been produced by re-cloning IgG mAbs, use of phage display derived antibodies, or by transgenic plant technology. Additionally, chemically-linked FcαRI bispecific antibodies (BsAb) have been used. The latter are generated from the backbones of two IgG mAbs that recognize either the extracellular domain of FcαRI or the antigen of interest. The generation of human IgA knock-in mice in which the first gene (encoding IgM) downstream of the joining genes is exchanged for a knock-in human Cα1 gene (α1KI mice) was recently described in reference Citation99. As such, homozygous α1KI mice produce human IgA instead of murine IgM, which will allow the generation of a continuous source of antigen-specific human IgA mAbs. The availability of these novel models combined with the existence of human FcαRI transgenic mice for testing will greatly facilitate future in vivo research.
IgA for prevention and treatment of infectious diseases.
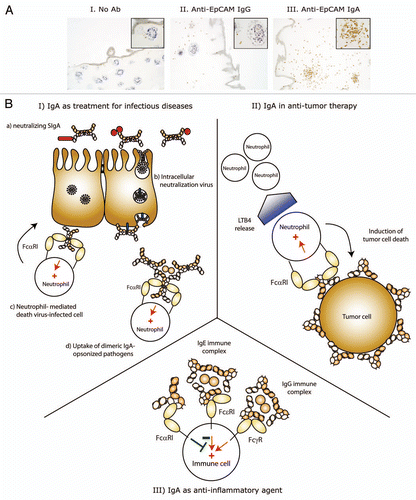
Mucosal sites, where IgA is the most prominent antibody class, are critical interfaces that separate the interior of the body and the outside world. At these sites, a fine balance must exist between mounting effective immunological defense against pathogenic microorganisms and avoiding responses against commensal microbial and environmental antigens.Citation11 SIgA plays an important role as the first line of defense by forming an anti-septic covering for the mucosa, hereby inhibiting adherence of microorganisms. Other mechanisms of displayed by SIgA include the ability to agglutinate microbes, interfere with bacterial motility by interacting with their flagella and neutralize bacterial products such as enzymes and toxins. Whereas antibodies usually offer little protection against intracellular pathogens, dimeric IgA has the intriguing ability to neutralize viruses intracellularly by intersecting virus particles and interfere with virus replication or assembly when in transit through an infected epithelial cell. IgA-virus complexes can subsequently be excreted into the lumen. Addition of specific anti-viral IgA to the basolateral surface of polarized epithelial cells was shown to reduce virus titers of Sendai virus, rotavirus, influenza and human immunodeficiency virus.Citation100–Citation104 Furthermore, polymeric IgA against toxin A of Clostridium difficile was able to prevent destruction of the epithelial monolayer.Citation105
Importantly, mice were protected against rotavirus, a diarrhea causing pathogen, when IgA mAbs were given systemically, but not when IgA was presented via the lumen of the intestinal tract, which supports the hypothesis that IgA transcytosis is required for viral inactivation in vivo.Citation106 Moreover, these data also suggest that systemically delivered IgA, transported via the pIgR route, is not hampered by locally produced mucosal IgA. Similarly, either passive transfer with specific IgA mAbs or oral immunization eliciting increased production of mucosal IgA was demonstrated to prevent Helicobacter felis, Helicobacter pylori,Citation107,Citation108 influenzaCitation109,Citation110 or Shigella flexneriCitation111 infection.
The protective effect of IgA is presumably even more pronounced in humans due to the presence of FcαRI. Both IgA that was purified from immune sera of patients with Bordetella pertussis infection and BsAb directed against Bordetella pertussis and FcαRI enhanced bacterial clearance in lungs of human FcαRI transgenic mice.Citation112 Furthermore, Escherichia coli bacteria that had been opsonised with human serum IgA were efficiently phagocytosed by FcαRI-expressing Kupffer cells in the liver of transgenic mice in vivo, which also supports a role for IgA in systemic clearance of pathogens.Citation45 It was recently demonstrated that passive transfer of human IgA mAbs against the α-crystallin of Mycobacterium tuberculosis protected human FcαRI transgenic mice, but not FcαRI-negative littermates against Mycobacterium tuberculosis infection.Citation113 Mycobacterium tuberculosis infection of human whole blood culture or isolated monocytes was reduced in the presence of IgA, albeit with high interdonor variability.
Neutrophilic granulocytes (neutrophils) are also likely involved in efficient IgA-mediated protective responses against pathogens. We recently demonstrated that monomeric and dimeric IgA have the unique ability to induce neutrophil migration directly,Citation75 whereas other antibody isotypes such as IgG and IgM induce neutrophil migration indirectly, through activation of the classical complement pathway (generating the chemoattractants C3a and C5a). However, after cross-linking of FcαRI, neutrophils release LTB4, which is a potent neutrophil chemoattractant. A self-contained neutrophil migration loop will thus be initiated until the infectious agent has been eliminated.
To date, enhanced uptake of Escherichia coli, Streptococcus pneumonia, Staphylococcus aureus, Porphyromonas gingivalis, Candida albicans, Bordetella Pertussis and Neisseria meningitidis by neutrophils in the presence of specific IgA or FcαRI BsAb targeting specific pathogens has been demonstrated.Citation45,Citation75,Citation112,Citation114–Citation116 Both monomeric and dimeric IgA proved effective in mediating phagocytosis by either neutrophils or Kupffer cells, but opsonic activity was reduced after binding of SC, which is consistent with a more anti-inflammatory role of SIgA.Citation45,Citation74 It was furthermore recently demonstrated that a specific anti-(gp41 x FcαRI) BsAb effectively directed neutrophils to destroy HIV-infected target cells.Citation117 Additionally, a BsAb targeting FcαRI and surfactant protein D, which demonstrated a broader binding to a great variety of pathogens via its carbohydrate recognition domain, induced uptake of Escherichia coli, Candida albicans and influenza virus by neutrophils.Citation118
Thus, therapies aimed to passively or actively increase specific IgA antibody titers against pathogens may significantly add to the arsenal of agents that fight (mucosal) infection. For instance, mucosal administration with transgenic plant SIgA afforded specific protection in humans against oral streptococcal colonization.Citation119 Furthermore, mucosal administration of an HIV-1 vaccine demonstrated both resistance to the virus and elicited virus-specific IgA with HIV-1 transcystosis-blocking properties in monkeys.Citation120
Targeting FcαRI for anti-tumor immunotherapy.
FcαRI was proposed as a novel trigger molecule for mAb-based anti-cancer therapy more than 10 years ago.Citation121,Citation122 However, because mice do not express an FcαRI homologue, it has proven difficult to test the efficacy of human IgA anti-tumor mAbs in vivo.Citation98,Citation123 This has seriously hampered the collection of in vivo data on the effects of targeting FcαRI. Nevertheless, in vitro experiments using therapeutic IgA1, IgA2, dimeric IgA, chimeric IgA and FcαRI BsAb targeting FcαRI have yielded promising results.Citation119,Citation121,Citation122,Citation124–Citation137 For instance, IgA mAbs were demonstrated to engage a different cell population as effector cells compared to IgG mAbs. It was demonstrated that neutrophils from healthy donors or (FcγRI-expressing) neutrophils from donors who had been treated with granulocyte-colony stimulating factor (G-CSF) triggered tumor cell killing much more effectively in the presence of anti-(HER2/neu x FcαRI) BsAb or anti-EpCAM IgA mAbs compared with an IgG counterpart. The superior ability of FcαRI to induce neutrophil-mediated tumor cell killing has now been demonstrated for a multitude of tumor-associated antigens, including HER2/neu (on breast carcinoma), EpCAM (colon carcinoma), EGFR (epithelial carcinoma and renal cell carcinoma), HLA class II (B-cell lymphoma), CD30 (T- and B-cell lymphoma) and carcinoembryonic antigen (CEA) in vitro. Notably, neutrophils were unable to kill malignant B cells via anti-CD20 IgG1 mAbs, but the addition of FcαRI targeting enabled this “antigen restriction” to be overcome, as tumor cells were efficiently killed in the presence of anti-(CD20 x FcαRI) BsAb.Citation121,Citation122,Citation124–Citation133,Citation135–Citation137
Furthermore, neutrophil accumulation and destruction of HER2/neu-expressing breast carcinoma colonies in a three-dimensional culture system was only observed in the presence of anti-(HER2/neu x FcαRI) BsAb, but not in the presence of a counterpart FcγRI BsAb.Citation132 Similar results were observed when colon carcinoma colonies were targeted with anti-EpCAM IgA, but not IgG mAb (), which is likely the result of LTB4 release after cross-linking of FcαRI.Citation75,Citation132 Enhanced neutrophil migration may therefore underlie increased ADCC after targeting with IgA mAb or FcαRI BsAb compared with IgG mAb or FcγR BsAb. However, it was additionally demonstrated that immature bone marrow neutrophils were not capable of killing tumor cells via FcγRI, whereas FcαRI efficiently induced ADCC.Citation134 Thus, alternatively the amplitude of signals mediated through FcαRI or FcγR may differ, since it was reported that interaction of FcαRI with FcRγ chain is stronger due to an electrostatic interaction that is absent for FcγR.Citation55
Treatment with G-CSF in order to mobilize neutrophils from the bone marrow combined with targeting FcαRI may improve clinical responses in cancer patients compared to results observed in unsuccessful trials that utilized therapeutic FcγRI BsAb to enlist neutrophils as effector cells. An additional attractive feature of recruiting neutrophils as effector cells is that targeting FcαRI on neutrophils induced necrotic and autophagic tumor cell death in vitro.Citation138 Because therapeutic IgG mAb facilitate natural killer (NK) cell-mediated apoptosis of tumor cells, targeting neutrophils as effector cells may represent a supplementary approach to kill tumor cells with mutations in apoptotic pathways.
Future Perspectives: IgA in Therapeutic Antibody Development!
Today, IgG mAbs are dominating the therapeutic antibody field because they have extended plasma half-life, efficiently activate complement and recruit NK cells for ADCC. Moreover, extensive time and effort is invested in optimizing IgG mAbs to fine-tune desired effector functions. Nonetheless, an accumulating amount of data indicates that IgA mAbs (or FcαRI BsAb) represent a promising addition to therapeutic strategies, especially in situations when IgG is less suitable, e.g., IgA responses will be likely more effective when active or passive mucosal immunity is required. Additionally, IgA mAbs are superior in recruiting neutrophils for antibody-mediated tumor cell killing, and may be a good alternative to overcome apoptosis resistance in tumor cells.
It must be noted that there are several challenges to the development of therapeutic IgA mAbs. First, the general miscomprehension of IgA as a non-inflammatory antibody needs to be rescinded, as many investigators have not really appreciated how powerful an inflammatory antibody IgA can be under the right circumstances. Second, adequate mouse models are urgently needed. The recent generation of human IgA knock-in mice and the availability of human FcαRI transgenic mice should greatly facilitate progress of therapeutic IgA mAb development. Third, the lack of established models for high IgA production and purification has hampered generation of IgA mAbs. However, the development of the human α1KI mouse model will allow hybridoma technology for specific human IgA production,Citation99 and advantages towards development of IgA-specific purification techniques have recently been established.Citation139–Citation141 The knowledge generated during optimizing IgG mAbs may furthermore significantly accelerate generation of effective IgA mAbs. For instance, IgA mAbs may require protein modulation to increase its plasma half-life, which is approximately a week.Citation142 This suggests less favourable pharmacokinetics compared to IgG, as the half-life of IgG is 1–3 weeks depending on the isotype. Another feature of IgA antibodies is the incapability of activating the classical complement pathway. This may be advantageous in certain situations, but may hamper therapeutic efficacy in other approaches. Since it has been demonstrated that the glycan moieties of IgA can activate the MBL complement pathway, glyco-engineering may be used to optimise IgA mAbs, although it may be challenging to manufacture heavily glycosylated proteins.
The functions of different IgA forms range from mere neutralization to active immune suppression or pro-inflammatory responses, and these differences can be exploited in the design and generation of IgA mAbs with specific desired therapeutic functional activity (). We thus anticipate that IgA mAbs will be a prominent part of the arsenal of therapeutic mAbs in the future.
Abbreviations
| ADCC | = | antibody dependent cellular cytotoxicity |
| Asn | = | asparagine |
| BsAb | = | bispecific antibodies |
| Cα | = | constant domain of IgA |
| CEA | = | carcinoembryonic antigen |
| DC | = | dendritic cell |
| dIgA | = | dimeric IgA |
| EC | = | extracellular domain |
| EGFR | = | epidermal growth factor receptor |
| EpCAM | = | epithelial cell adhesion molecule |
| FcR | = | Fc receptor |
| G-CSF | = | granulocyte colony stimulating factor |
| HER2/neu | = | human epidermal growth factor receptor 2 |
| IgA | = | immunoglobulin A |
| IL | = | interleukin |
| ITAM | = | immunoreceptor tyrosine based activation motif |
| KI | = | knock-in |
| KIR | = | killer inhibitory receptors |
| LIR | = | leukocyte Ig-like receptors |
| LRC | = | leukocyte receptor cluster |
| mAb | = | monoclonal antibody |
| PI3K | = | phosphatidylinositol-3-kinase |
| pIgR | = | polymeric Ig receptor |
| PKB | = | protein kinase B |
| PKC | = | protein kinase C |
| PLC | = | phospholipase C |
| PP2A | = | protein phosphatase 2A |
| SHP−1 | = | protein tyrosine phosphatase |
| sIgA | = | secretory IgA |
| SSL | = | staphylococcal superantigen-like proteins |
| Syk | = | spleen tyrosine kinase |
Figures and Tables
Figure 1 Schematic model of (A) monomeric human IgA1, IgA2m(1) and IgA2m(2), (B) dimeric IgA2 and (C) secretory IgA2. Heavy chains are depicted in light and dark orange, whereas light chains are shown in brown. J-chains or secretory component (SC) are indicated by blue or red, respectively. IgA1 contains O-linked oligosaccharides in the hinge region, which are depicted as white circles, whereas N-linked oligosaccharides are shown as black circles. For clarity, glycosylation of J-chain and secretory component has been omitted.
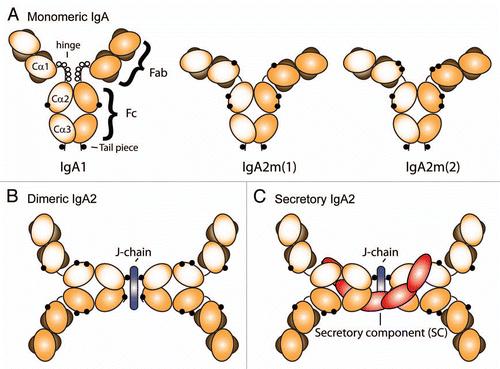
Figure 2 FcαRI initiates different functional outcome depending on the mode of interaction with its ligand IgA or FcRγ chain. (1) Functionality of FcαRI “γ-less” receptors is limited to ligand binding and receptor internalization (although Gly248-FcαRI can trigger IL-6 production as well). (2) Cross-linking of FcαRI via IgA immune complexes (IC) leads to heavily phosphorylated (p) FcRγ chain ITAMs, which engage Syk. This results in activation and pro-inflammatory responses. (3) Binding of monomeric IgA to FcαRI mediates weaker phosphorylation of FcR γ chain ITAM and recruitment of SHP-1. This elicits an inhibitory signal, which can suppress activation through other Fc receptors.
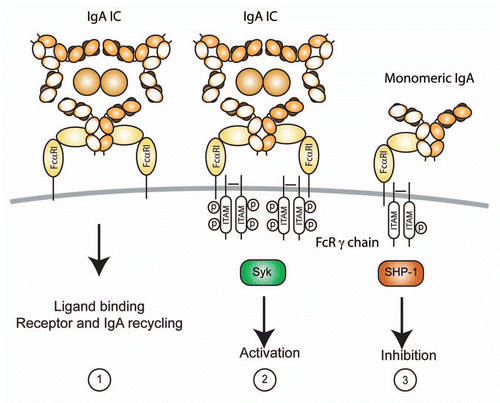
Figure 3 Therapeutic potential of IgA mAbs. (A) Interferon-γ stimulated neutrophils (to induced FcγRI expression and delay neutrophil apoptosis) were added to human colon carcinoma colonies in collagen in the absence (part I) or presence of anti-EpCAM IgG (part II) or IgA (part III) mAbs. After 24 h, collagen gels were fixed and slides were stained for CD66b (neutrophil marker, brown staining). Only anti-EpCAM IgA mAb induced neutrophil migration in and destruction of tumor colonies. (B) Schematic representation of therapeutic strategies using IgA mAbs. (I) Treatment of infectious diseases: (a) Passive mucosal immunization with SIgA can prevent infection and neutralize bacterial toxins. Systemic treatment with dimeric IgA or active mucosal immunization to elicit increased IgA responses can lead to (b) intracellular virus neutralization and neutrophil mediated killing of (c) virus-infected cells or (d) dimeric IgA-opsonized pathogens (bacteria, fungi). (II) IgA mAbs are not only superior in inducing tumor cell death, but also recruit neutrophils, since FcαRI cross-linking leads to LTB4 release. (III) The ability of monomeric IgA to inhibit immune responses (initiated by e.g., IgE or IgG complexes) was demonstrated in murine asthma and kidney inflammation models,Citation89,Citation90,Citation143 which supports the use of monomeric IgA IVIG treatment to prevent or reverse established inflammatory diseases. (+, activation; −, inhibition).
Acknowledgments
J.E. Bakema is supported by the Netherlands Organization for Scientific Research (VIDI 016.086.320).
References
- Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol 2006; 208:270 - 282
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 2000; 288:2222 - 2226
- Maliszewski CR, March CJ, Schoenborn MA, Gimpel S, Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med 1990; 172:1665 - 1672
- Morton HC, van Egmond M, van de Winkel JG. Structure and function of human IgA Fc receptors (Fc alpha R). Crit Rev Immunol 1996; 16:423 - 440
- Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory ITAMs as novel regulators of immunity. Immunol Rev 2009; 232:59 - 71
- Monteiro RC. The role of IgA and IgA Fc receptors as anti-inflammatory agents. J Clin Immunol 2010; 30:61 - 64
- Snoeck V, Peters IR, Cox E. The IgA system: a comparison of structure and function in different species. Vet Res 2006; 37:455 - 467
- Lewis MJ, Wagner B, Irvine RM, Woof JM. IgA in the horse: cloning of equine polymeric Ig receptor and J chain and characterization of recombinant forms of equine IgA. Mucosal Immunol 2010; 3:610 - 621
- Kerr MA. The structure and function of human IgA. Biochem J 1990; 271:285 - 296
- Delacroix DL, Dive C, Rambaud JC, Vaerman JP. IgA subclasses in various secretions and in serum. Immunology 1982; 47:383 - 385
- van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JG. IgA and the IgA Fc receptor. Trends Immunol 2001; 22:205 - 211
- Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen EV. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. Apmis 1996; 104:321 - 338
- Tomana M, Niedermeier W, Mestecky J, Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry 1976; 13:325 - 328
- Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, et al. The glycosylation and structure of human serum IgA1, Fab and Fc regions and the role of N-glycosylation on Fcalpha receptor interactions. J Biol Chem 1998; 273:2260 - 2272
- Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem 2003; 278:20140 - 20153
- Tarelli E, Smith AC, Hendry BM, Challacombe SJ, Pouria S. Human serum IgA1 is substituted with up to six O-glycans as shown by matrix assisted laser desorption ionisation time-of-flight mass spectrometry. Carbohydr Res 2004; 339:2329 - 2335
- Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 2001; 167:2861 - 2868
- Boehm MK, Woof JM, Kerr MA, Perkins SJ. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: a study by X-ray and neutron solution scattering and homology modelling. J Mol Biol 1999; 286:1421 - 1447
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol 1994; 12:63 - 84
- Johansen FE, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J Immunol 2001; 167:5185 - 5192
- Braathen R, Sorensen V, Brandtzaeg P, Sandlie I, Johansen FE. The carboxyl-terminal domains of IgA and IgM direct isotype-specific polymerization and interaction with the polymeric immunoglobulin receptor. J Biol Chem 2002; 277:42755 - 42762
- Lewis MJ, Pleass RJ, Batten MR, Atkin JD, Woof JM. Structural requirements for the interaction of human IgA with the human polymeric Ig receptor. J Immunol 2005; 175:6694 - 6701
- Norderhaug IN, Johansen FE, Schjerven H, Brandtzaeg P. Regulation of the formation and external transport of secretory immunoglobulins. Crit Rev Immunol 1999; 19:481 - 508
- Lawrence DA, Weigle WO, Spiegelberg HL. Immunoglobulins cytophilic for human lymphocytes, monocytes and neutrophils. J Clin Invest 1975; 55:368 - 376
- Wines BD, Hogarth PM. IgA receptors in health and disease. Tissue Antigens 2006; 68:103 - 114
- Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol 2000; 1:441 - 446
- Stockert RJ, Kressner MS, Collins JC, Sternlieb I, Morell AG. IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci USA 1982; 79:6229 - 6231
- Moura IC, Centelles MN, Arcos-Fajardo M, Malheiros DM, Collawn JF, Cooper MD, et al. Identification of the transferrin receptor as a novel immunoglobulin (Ig) A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med 2001; 194:417 - 425
- Lamkhioued B, Gounni AS, Gruart V, Pierce A, Capron A, Capron M. Human eosinophils express a receptor for secretory component. Role in secretory IgA-dependent activation. Eur J Immunol 1995; 25:117 - 125
- Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J Immunol 2002; 169:1844 - 1851
- Kazeeva TN, Shevelev AB. IgA-specific proteins of pathogenic bacteria. Biochemistry (Mosc) 2009; 74:12 - 21
- Kremer EJ, Kalatzis V, Baker E, Callen DF, Sutherland GR, Maliszewski CR. The gene for the human IgA Fc receptor maps to 19q13.4. Hum Genet 1992; 89:107 - 108
- Martin AM, Kulski JK, Witt C, Pontarotti P, Christiansen FT. Leukocyte Ig-like receptor complex (LRC) in mice and men. Trends Immunol 2002; 23:81 - 88
- Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol 2003; 21:177 - 204
- Maruoka T, Nagata T, Kasahara M. Identification of the rat IgA Fc receptor encoded in the leukocyte receptor complex. Immunogenetics 2004; 55:712 - 716
- Morton HC, Pleass RJ, Storset AK, Dissen E, Williams JL, Brandtzaeg P, et al. Cloning and characterization of an immunoglobulin A Fc receptor from cattle. Immunology 2004; 111:204 - 211
- Morton HC, Pleass RJ, Storset AK, Brandtzaeg P, Woof JM. Cloning and characterization of equine CD89 and identification of the CD89 gene in chimpanzees and rhesus macaques. Immunology 2005; 115:74 - 84
- Morton HC. IgA Fc receptors in cattle and horses. Vet Immunol Immunopathol 2005; 108:139 - 143
- Reljic R. In search of the elusive mouse macrophage Fc-alpha receptor. Immunol Lett 2006; 107:80 - 81
- Sibille Y, Chatelain B, Staquet P, Delacroix DL, Vaerman JP. IgA receptors on human alveolar macrophages. Monogr Allergy 1988; 24:282 - 286
- Monteiro RC, Kubagawa H, Cooper MD. Cellular distribution, regulation and biochemical nature of an Fc alpha receptor in humans. J Exp Med 1990; 171:597 - 613
- Monteiro RC, Cooper MD, Kubagawa H. Molecular heterogeneity of Fc alpha receptors detected by receptor-specific monoclonal antibodies. J Immunol 1992; 148:1764 - 1770
- Monteiro RC, Hostoffer RW, Cooper MD, Bonner JR, Gartland GL, Kubagawa H. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest 1993; 92:1681 - 1685
- Patry C, Sibille Y, Lehuen A, Monteiro RC. Identification of Fcalpha receptor (CD89) isoforms generated by alternative splicing that are differentially expressed between blood monocytes and alveolar macrophages. J Immunol 1996; 156:4442 - 4448
- van Egmond M, van Garderen E, van Spriel AB, Damen CA, van Amersfoort ES, van Zandbergen G, et al. FcalphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med 2000; 6:680 - 685
- Geissmann F, Launay P, Pasquier B, Lepelletier Y, Leborgne M, Lehuen A, et al. A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J Immunol 2001; 166:346 - 352
- Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are downregulated for LPS- and IgA-mediated activities. J Immunol 2001; 167:2651 - 2656
- Heystek HC, Moulon C, Woltman AM, Garonne P, van Kooten C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J Immunol 2002; 168:102 - 107
- Qian K, Xie F, Gibson AW, Edberg JC, Kimberly RP, Wu J. Functional expression of IgA receptor FcalphaRI on human platelets. J Leukoc Biol 2008; 84:1492 - 1500
- Chevailler A, Monteiro RC, Kubagawa H, Cooper MD. Immunofluorescence analysis of IgA binding by human mononuclear cells in blood and lymphoid tissue. J Immunol 1989; 142:2244 - 2249
- Bakema JE, Hiemstra IH, Bakker J, de Haij S, Kok Y, Adema G, et al. c-Jun activating binding protein 1 binds to the IgA receptor and modulates protein levels of FcalphaRI and FcRgamma-chain. Eur J Immunol 2010; 40:2035 - 2040
- Hostoffer RW, Krukovets I, Berger M. Increased Fc alpha R expression and IgA-mediated function on neutrophils induced by chemoattractants. J Immunol 1993; 150:4532 - 4540
- Yin N, Peng M, Xing Y, Zhang W. Intracellular pools of FcalphaR (CD89) in human neutrophils are localized in tertiary granules and secretory vesicles and two FcalphaR isoforms are found in tertiary granules. J Leukoc Biol 2007; 82:551 - 558
- Pfefferkorn LC, Yeaman GR. Association of IgA-Fc receptors (Fc alpha R) with Fc epsilon RI gamma2 subunits in U937 cells. Aggregation induces the tyrosine phosphorylation of gamma2. J Immunol 1994; 153:3228 - 3236
- Morton HC, van den Herik-Oudijk IE, Vossebeld P, Snijders A, Verhoeven AJ, Capel PJ, et al. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcRgamma chain. Molecular basis for CD89/FcRgamma chain association. J Biol Chem 1995; 270:29781 - 29787
- Wines BD, Trist HM, Monteiro RC, Van Kooten C, Hogarth PM. Fc receptor gamma chain residues at the interface of the cytoplasmic and transmembrane domains affect association with FcalphaRI, surface expression and function. J Biol Chem 2004; 279:26339 - 26345
- Bakema JE, de Haij S, den Hartog-Jager CF, Bakker J, Vidarsson G, van Egmond M, et al. Signaling through mutants of the IgA receptor CD89 and consequences for Fc receptor gamma-chain interaction. J Immunol 2006; 176:3603 - 3610
- Wines BD, Trist HM, Ramsland PA, Hogarth PM. A common site of the Fc receptor gamma subunit interacts with the unrelated immunoreceptors FcalphaRI and FcepsilonRI. J Biol Chem 2006; 281:17108 - 17113
- van Dijk TB, Bracke M, Caldenhoven E, Raaijmakers JA, Lammers JW, Koenderman L, et al. Cloning and characterization of Fcalpha Rb, a novel Fcalpha receptor (CD89) isoform expressed in eosinophils and neutrophils. Blood 1996; 88:4229 - 4238
- Jasek M, Manczak M, Sawaryn A, Obojski A, Wisniewski A, Luszczek W, et al. A novel polymorphism in the cytoplasmic region of the human immunoglobulin A Fc receptor gene. Eur J Immunogenet 2004; 31:59 - 62
- Ding Y, Xu G, Yang M, Yao M, Gao GF, Wang L, et al. Crystal structure of the ectodomain of human FcalphaRI. J Biol Chem 2003; 278:27966 - 27970
- Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature 2003; 423:614 - 620
- Woof JM, Burton DR. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol 2004; 4:89 - 99
- Wines BD, Hulett MD, Jamieson GP, Trist HM, Spratt JM, Hogarth PM. Identification of residues in the first domain of human Fcalpha receptor essential for interaction with IgA. J Immunol 1999; 162:2146 - 2153
- Wines BD, Sardjono CT, Trist HH, Lay CS, Hogarth PM. The interaction of FcalphaRI with IgA and its implications for ligand binding by immunoreceptors of the leukocyte receptor cluster. J Immunol 2001; 166:1781 - 1789
- Xue J, Zhao Q, Zhu L, Zhang W. Deglycosylation of FcalphaR at N58 increases its binding to IgA. Glycobiology 2010; 20:905 - 915
- Lu J, Marjon KD, Marnell LL, Wang R, Mold C, Du Clos TW, et al. Recognition and functional activation of the human IgA receptor (Fc{alpha}RI) by C-reactive protein. Proc Natl Acad Sci USA 2011; 108:4974 - 4979
- Shi J, Ghirlando R, Beavil RL, Beavil AJ, Keown MB, Young RJ, et al. Interaction of the low-affinity receptor CD23/Fc epsilonRII lectin domain with the Fc epsilon3-4 fragment of human immunoglobulin E. Biochemistry 1997; 36:2112 - 2122
- Keown MB, Ghirlando R, Mackay GA, Sutton BJ, Gould HJ. Basis of the 1:1 stoichiometry of the high affinity receptor Fcepsilon RI-IgE complex. Eur Biophys J 1997; 25:471 - 476
- Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 2000; 406:267 - 273
- Keown MB, Henry AJ, Ghirlando R, Sutton BJ, Gould HJ. Thermodynamics of the interaction of human immunoglobulin E with its high-affinity receptor Fc epsilon RI. Biochemistry 1998; 37:8863 - 8869
- Zhang Y, Boesen CC, Radaev S, Brooks AG, Fridman WH, Sautes-Fridman C, et al. Crystal structure of the extracellular domain of a human Fcgamma RIII. Immunity 2000; 13:387 - 395
- van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, et al. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 2001; 97:2478 - 2486
- Vidarsson G, van Der Pol WL, van Den Elsen JM, Vile H, Jansen M, Duijs J, et al. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J Immunol 2001; 166:6250 - 6256
- van der Steen L, Tuk CW, Bakema JE, Kooij G, Reijerkerk A, Vidarsson G, et al. Immunoglobulin A: Fc(alpha)RI interactions induce neutrophil migration through release of leukotriene B4. Gastroenterology 2009; 137:2018 - 2029
- Oortwijn BD, Roos A, van der Boog PJ, Klar-Mohamad N, van Remoortere A, Deelder AM, et al. Monomeric and polymeric IgA show a similar association with the myeloid FcalphaRI/CD89. Mol Immunol 2007; 44:966 - 973
- Pleass RJ, Dunlop JI, Anderson CM, Woof JM. Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human fcalpha receptor (FcalphaR) CD89. J Biol Chem 1999; 274:23508 - 23514
- Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB. Analysis of IgA1 N-glycosylation and its contribution to FcalphaRI binding. Biochemistry 2008; 47:11285 - 11299
- Reterink TJ, van Zandbergen G, van Egmond M, Klar-Mohamad N, Morton CH, van de Winkel JG, et al. Size-dependent effect of IgA on the IgA Fc receptor (CD89). Eur J Immunol 1997; 27:2219 - 2224
- Baldwin GC, Gasson JC, Quan SG, Fleischmann J, Weisbart R, Oette D, et al. Granulocyte-macrophage colony-stimulating factor enhances neutrophil function in acquired immunodeficiency syndrome patients. Proc Natl Acad Sci USA 1988; 85:2763 - 2766
- Bracke M, Dubois GR, Bolt K, Bruijnzeel PL, Vaerman JP, Lammers JW, et al. Differential effects of the T helper cell type 2-derived cytokines IL-4 and IL-5 on ligand binding to IgG and IgA receptors expressed by human eosinophils. J Immunol 1997; 159:1459 - 1465
- Bracke M, Lammers JW, Coffer PJ, Koenderman L. Cytokine-induced inside-out activation of FcalphaR (CD89) is mediated by a single serine residue (S263) in the intracellular domain of the receptor. Blood 2001; 97:3478 - 3483
- Bakema JE, Bakker A, de Haij S, Honing H, Bracke M, Koenderman L, et al. Inside-out regulation of FcalphaRI (CD89) depends on PP2A. J Immunol 2008; 181:4080 - 4088
- van Egmond M, van Vuuren AJ, Morton HC, van Spriel AB, Shen L, Hofhuis FM, et al. Human immunoglobulin A receptor (FcalphaRI, CD89) function in transgenic mice requires both FcRgamma chain and CR3 (CD11b/CD18). Blood 1999; 93:4387 - 4394
- Launay P, Patry C, Lehuen A, Pasquier B, Blank U, Monteiro RC. Alternative endocytic pathway for immunoglobulin A Fc receptors (CD89) depends on the lack of FcRgamma association and protects against degradation of bound ligand. J Biol Chem 1999; 274:7216 - 7225
- Honorio-Franca AC, Launay P, Carneiro-Sampaio MM, Monteiro RC. Colostral neutrophils express Fc alpha receptors (CD89) lacking gamma chain association and mediate noninflammatory properties of secretory IgA. J Leukoc Biol 2001; 69:289 - 296
- Shen L, van Egmond M, Siemasko K, Gao H, Wade T, Lang ML, et al. Presentation of ovalbumin internalized via the immunoglobulin-A Fc receptor is enhanced through Fc receptor gamma-chain signaling. Blood 2001; 97:205 - 213
- Wu J, Ji C, Xie F, Langefeld CD, Qian K, Gibson AW, et al. FcalphaRI (CD89) alleles determine the proinflammatory potential of serum IgA. J Immunol 2007; 178:3973 - 3982
- Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffie C, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity 2005; 22:31 - 42
- Kanamaru Y, Blank U, Monteiro RC. IgA Fc receptor I is a molecular switch that determines IgA activating or inhibitory functions. Contrib Nephrol 2007; 157:148 - 152
- Van Epps DE, Brown SL. Inhibition of formylmethionyl-leucyl-phenylalanine-stimulated neutrophil chemiluminescence by human immunoglobulin A paraproteins. Infect Immun 1981; 34:864 - 870
- Van Epps DE, Williams R Jr. Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med 1976; 144:1227 - 1242
- Van Epps DE, Reed K, Williams R Jr. Suppression of human PMN bactericidal activity by human IgA paraproteins. Cell Immunol 1978; 36:363 - 376
- Wilton JM. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol 1978; 34:423 - 428
- Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 1994; 83:1278 - 1288
- Wolf HM, Eibl MM. The anti-inflammatory effect of an oral immunoglobulin (IgA-IgG) preparation and its possible relevance for the prevention of necrotizing enterocolitis. Acta Paediatr Suppl 1994; 396:37 - 40
- Nikolova EB, Russell MW. Dual function of human IgA antibodies: inhibition of phagocytosis in circulating neutrophils and enhancement of responses in IL-8-stimulated cells. J Leukoc Biol 1995; 57:875 - 882
- van Egmond M, Hanneke van Vuuren AJ, van de Winkel JG. The human Fc receptor for IgA (FcalphaRI, CD89) on transgenic peritoneal macrophages triggers phagocytosis and tumor cell lysis. Immunol Lett 1999; 68:83 - 87
- Duchez S, Amin R, Cogne N, Delpy L, Sirac C, Pascal V, et al. Premature replacement of mu with alpha immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proc Natl Acad Sci USA 2010; 107:3064 - 3069
- Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA 1992; 89:6901 - 6905
- Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol 1995; 69:1339 - 1343
- Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Peterra J, Nedrud JG. Intracellular neutralization of Sendai and influenza viruses by IgA monoclonal antibodies. Adv Exp Med Biol 1995; 371:651 - 654
- Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol 2000; 165:5170 - 5176
- Corthesy B, Benureau Y, Perrier C, Fourgeux C, Parez N, Greenberg H, et al. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol 2006; 80:10692 - 10699
- Stubbe H, Berdoz J, Kraehenbuhl JP, Corthesy B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J Immunol 2000; 164:1952 - 1960
- Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 1996; 272:104 - 107
- Blanchard TG, Czinn SJ, Maurer R, Thomas WD, Soman G, Nedrud JG. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect Immun 1995; 63:1394 - 1399
- Goto T, Nishizono A, Fujioka T, Ikewaki J, Mifune K, Nasu M. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect Immun 1999; 67:2531 - 2539
- Renegar KB, Small P Jr. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol 1991; 146:1972 - 1978
- Takase H, Murakami Y, Endo A, Ikeuchi T. Antibody responses and protection in mice immunized orally against influenza virus. Vaccine 1996; 14:1651 - 1656
- Phalipon A, Kaufmann M, Michetti P, Cavaillon JM, Huerre M, Sansonetti P, et al. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med 1995; 182:769 - 778
- Hellwig SM, van Spriel AB, Schellekens JF, Mooi FR, van de Winkel JG. Immunoglobulin A-mediated protection against Bordetella pertussis infection. Infect Immun 2001; 69:4846 - 4850
- Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol 2011; 186:3113 - 3119
- van Spriel AB, van den Herik-Oudijk IE, van Sorge NM, Vile HA, van Strijp JA, van de Winkel JG. Effective phagocytosis and killing of Candida albicans via targeting FcgammaRI (CD64) or FcalphaRI (CD89) on neutrophils. J Infect Dis 1999; 179:661 - 669
- van der Pol W, Vidarsson G, Vile HA, van de Winkel JG, Rodriguez ME. Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via FcalphaRI (CD89). J Infect Dis 2000; 182:1139 - 1145
- Kobayashi T, Yamamoto K, Sugita N, van Spriel AB, Kaneko S, van de Winkel JG, et al. Effective in vitro clearance of Porphyromonas gingivalis by Fcalpha receptor I (CD89) on gingival crevicular neutrophils. Infect Immun 2001; 69:2935 - 2942
- Duval M, Posner MR, Cavacini LA. A bispecific antibody composed of a nonneutralizing antibody to the gp41 immunodominant region and an anti-CD89 antibody directs broad human immunodeficiency virus destruction by neutrophils. J Virol 2008; 82:4671 - 4674
- Tacken PJ, Hartshorn KL, White MR, van Kooten C, van de Winkel JG, Reid KB, et al. Effective targeting of pathogens to neutrophils via chimeric surfactant protein D/anti-CD89 protein. J Immunol 2004; 172:4934 - 4940
- Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med 1998; 4:601 - 606
- Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 2011; 34:269 - 280
- Valerius T, Stockmeyer B, van Spriel AB, Graziano RF, van den Herik-Oudijk IE, Repp R, et al. FcalphaRI (CD89) as a novel trigger molecule for bispecific antibody therapy. Blood 1997; 90:4485 - 4492
- Deo YM, Sundarapandiyan K, Keler T, Wallace PK, Graziano RF. Bispecific molecules directed to the Fc receptor for IgA (FcalphaRI, CD89) and tumor antigens efficiently promote cell-mediated cytotoxicity of tumor targets in whole blood. J Immunol 1998; 160:1677 - 1686
- Launay P, Grossetete B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, et al. Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger's disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 2000; 191:1999 - 2009
- Valerius T, Wurflein D, Stockmeyer B, Repp R, Kalden JR, Gramatzki M. Activated neutrophils as effector cells for bispecific antibodies. Cancer Immunol Immunother 1997; 45:142 - 145
- Huls G, Heijnen IA, Cuomo E, van der Linden J, Boel E, van de Winkel JG, et al. Antitumor immune effector mechanisms recruited by phage display-derived fully human IgG1 and IgA1 monoclonal antibodies. Cancer Res 1999; 59:5778 - 5784
- Stockmeyer B, Dechant M, van Egmond M, Tutt AL, Sundarapandiyan K, Graziano RF, et al. Triggering Fc alpha-receptor I (CD89) recruits neutrophils as effector cells for CD20-directed antibody therapy. J Immunol 2000; 165:5954 - 5961
- Dechant M, Valerius T. IgA antibodies for cancer therapy. Crit Rev Oncol Hematol 2001; 39:69 - 77
- Stockmeyer B, Elsasser D, Dechant M, Repp R, Gramatzki M, Glennie MJ, et al. Mechanisms of G-CSF- or GM-CSF-stimulated tumor cell killing by Fc receptor-directed bispecific antibodies. J Immunol Methods 2001; 248:103 - 111
- Sundarapandiyan K, Keler T, Behnke D, Engert A, Barth S, Matthey B, et al. Bispecific antibody-mediated destruction of Hodgkin's lymphoma cells. J Immunol Methods 2001; 248:113 - 123
- van Egmond M, van Spriel AB, Vermeulen H, Huls G, van Garderen E, van de Winkel JG. Enhancement of polymorphonuclear cell-mediated tumor cell killing on simultaneous engagement of fcgammaRI (CD64) and fcalphaRI (CD89). Cancer Res 2001; 61:4055 - 4060
- Dechant M, Vidarsson G, Stockmeyer B, Repp R, Glennie MJ, Gramatzki M, et al. Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood 2002; 100:4574 - 4580
- Otten MA, Rudolph E, Dechant M, Tuk CW, Reijmers RM, Beelen RH, et al. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol 2005; 174:5472 - 5480
- Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JG, et al. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol 2007; 179:2936 - 2943
- Otten MA, Leusen JH, Rudolph E, van der Linden JA, Beelen RH, van de Winkel JG, et al. FcRgamma-chain dependent signaling in immature neutrophils is mediated by FcalphaRI, but not by FcgammaRI. J Immunol 2007; 179:2918 - 2924
- Zhao J, Kuroki M, Shibaguchi H, Wang L, Huo Q, Takami N, et al. Recombinant human monoclonal igA antibody against CEA to recruit neutrophils to CEA-expressing cells. Oncol Res 2008; 17:217 - 222
- Guettinger Y, Barbin K, Peipp M, Bruenke J, Dechant M, Horner H, et al. A recombinant bispecific single-chain fragment variable specific for HLA class II and FcalphaRI (CD89) recruits polymorphonuclear neutrophils for efficient lysis of malignant B lymphoid cells. J Immunol 2010; 184:1210 - 1217
- Lohse S, Derer S, Beyer T, Klausz K, Peipp M, Leusen JH, et al. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J Immunol 2011; 186:3770 - 3778
- Bakema JE, Ganzevles SH, Fluitsma DM, Schilham MW, Beelen RHJ, Valerius T, et al. Targeting the immunoglobulin A Fc receptor (FcalphaRI) on polymorphonuclear cells induces tumor cell killing through autophagy. J Immunol 2011; 187:726 - 732
- Leibl H, Tomasits R, Mannhalter JW. Isolation of human serum IgA using thiophilic adsorption chromatography. Protein Expr Purif 1995; 6:408 - 410
- Sandin C, Linse S, Areschoug T, Woof JM, Reinholdt J, Lindahl G. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J Immunol 2002; 169:1357 - 1364
- Beyer T, Lohse S, Berger S, Peipp M, Valerius T, Dechant M. Serum-free production and purification of chimeric IgA antibodies. J Immunol Methods 2009; 346:26 - 37
- Morell A, Skvaril F, Noseda G, Barandun S. Metabolic properties of human IgA subclasses. Clin Exp Immunol 1973; 13:521 - 528
- Kanamaru Y, Pfirsch S, Aloulou M, Vrtovsnik F, Essig M, Loirat C, et al. Inhibitory ITAM signaling by FcalphaRI-FcRgamma chain controls multiple activating responses and prevents renal inflammation. J Immunol 2008; 180:2669 - 2678