Abstract
Bacteroides spp. organisms, the predominant commensal bacteria in the human gut have become increasingly resistant to many antibiotics. They are now also considered to be reservoirs of antibiotic resistance genes due to their capacity to harbor and disseminate these genes via mobile transmissible elements that occur in bewildering variety. Gene dissemination occurs within and from Bacteroides spp. primarily by conjugation, the molecular mechanisms of which are still poorly understood in the genus, even though the need to prevent this dissemination is urgent. One current avenue of research is thus focused on interventions that use non-antibiotic methodologies to prevent conjugation-based DNA transfer.
Introduction
It has been estimated that the human microflora collectively number up to 100 trillion cells, 10-fold the number of human cells.Citation1,Citation2 The majority of these normal flora reside in the gastrointestinal tract, and comprise over 500 bacterial species that exert a profound influence on human physiology.Citation3 Of these bacteria, 99% are anaerobes.Citation4 Anaerobic bacteroidetes are the predominant class, accounting about 30% of all bacteria in the human gut.Citation5,Citation6
Bacteroides spp are bile-resistant, non-spore-forming, gram-negative, rod-shaped anaerobes that are a dominant bacterial genus in the human colon and are less abundant in the intestines of other animals and in the environment.Citation7,Citation8 Bacteroides sp are passed from mother to child during vaginal birth and thus become part of the human flora in the earliest stages of life.Citation9 The C+G nucleotide composition of the Bacteroides genome is in the range of 40–48%. Its membranes contain sphingolipids, which are unusual in bacteria.
As an integral part of the normal human gut flora, Bacteroides spp play a number of roles, such as providing energy for the host in the form of short-chain fatty acids and sugars, recycling bile acids, and aiding in the development of the host immune system.Citation6 They also exhibit unique adaptations to successfully colonize the gut such as the ability to change their cell surface architecture,Citation10 ability to stimulate host expression of fucosylated glycoproteins as well as synthesize them,Citation11 and the ability to tolerate and use oxygen.Citation12
However, when Bacteroides spp escape the gut due to surgery, trauma or disease, they can cause life-threatening infections such as peritonitis and intra-abdominal sepsis.Citation6,Citation8,Citation13 Bacteroides spp rarely cause endocarditis, inflammation of the inner layer of the heart, but when it does occur, it can be serious with a mortality rate of 21–43%,Citation14 and increase hospital stays by up to 15 days.Citation15 These organisms can also be associated with other infections such as those of the skin, soft tissue, joints (septic arthritis), and brain (abscesses and meningitis).Citation6 Among more than 20 species of the genus, B. fragilis is most frequently isolated from clinical specimens, followed by B. ovatus and B. thetaiotaomicron.Citation6,Citation16,Citation17 Enterotoxigenic Bacteroides fragilis (ETBF; a sub-group of B. fragilis) has also been implicated in inflammatory bowel disease (IBD)Citation18,Citation19 and colon cancer.Citation20 Recently, a 5 y study in Japan revealed that Bacteroides bacteremia was the primary cause of colorectal carcinoma in a group of patients.Citation21 Bacteroides spp are thus among the most commonly isolated anaerobic pathogens,Citation19 and it is now appreciated that this is likely due to the expression of diverse virulence factors including surface polysaccharide capsules, outer membrane vesicles, toxins and β-lactamases.Citation22 In addition, the capacity of B. fragilis to tolerate nanomolar concentrations of oxygen allows this species to predominate in infections of the peritoneal cavity.
Antimicrobial resistance in the Bacteroides further complicates the clinical picture. Many Bacteroides spp are resistant to aminoglycosides (gentamicin, kanamycin, streptomycin), tetracycline (nearly 85% of clinical isolates), β-lactam antibiotics (penicillin, ampicillin, cephalosporins, cefoxitin, cephamycins and carbapenems), metronidazole and the macrolide-lincosamide-streptogramin (MLS) group of antibiotics (erythromycin and clindamycin).Citation7,Citation8 Of concern, all of these resistance traits have been found on transmissible genetic elements obviously contributing to resistance gene dissemination.Citation8 Over the past decades, carriage of the tetracycline resistance gene, tetQ, has increased from about 30% to more than 80% in clinical strains.Citation8 Specifically, over the past 10 y, resistance of Bacteroides spp has increased dramatically worldwide, especially to commonly prescribed antibiotics such as clindamycin and the cephalosporins.Citation15,Citation23–Citation26 Bacteroides spp resistance to fluoroquinolones has also increased from 1.5–12% during the past few years.Citation27,Citation28 Metronidazole-resistant strains of B. fragilis have been reported in many countries, including Brazil,Citation29 India,Citation30 United States,Citation31 HungaryCitation32 and Poland,Citation33 and some Indian strains show very high levels of resistance (MIC 512 mg/L).Citation34 Therefore, anaerobic infections have reemerged as a serious health threat, and Bacteroides spp are now recognized as important pathogens.
Mobile Genetic Elements in the Bacteroides
Bacteroides spp harbor many conjugative and mobilizable elements (). Conjugative elements are autonomous and self-transferable, i.e., they encode all functions for DNA transfer, including those resulting in nucleic acid processing (DNA transfer initiation) as well as conjugation channel/portal assembly and DNA translocation (mating apparatus formation). Mobilizable elements encode only DNA processing functions (), and use (or “hijack”) the mating apparatus of co-resident conjugative element(s) to transfer to recipient bacteria. Interestingly, in the Bacteroides, a wide variety of mobilizable elements appear to be able to translocate through the same mating apparatus encoded by a single conjugative element. A significant proportion of Bacteroides mobile elements also harbor antibiotic resistance genes, and are thus responsible for the widespread dissemination of those genes. Both conjugative and mobilizable elements can be transposons or plasmids.
Plasmids.
Plasmids are very common in Bacteroides spp and are found in 20–50% of strains.Citation6 Plasmids can replicate as independent elements in the host bacteria, and some can integrate into the host genome.Citation35 Many plasmids also have an origin of transfer (oriT) and a trans-acting mobilization gene, which allow them to be transferred by conjugation.
Antibiotic resistance genes have been found on plasmids in Bacteroides spp Genes whose products confer resistance to metronidazole, chloramphenicol, carbapenems, clindamycin and erythromycin have been found on mobile plasmids from Bacteroides spp clinical isolates worldwide. Resistance genes nimA-nimF, encoding metronidazole resistance, have alos been identified on transferrable plasmids and recovered worldwide.Citation36 The cfiA gene, conferring resistance to carbapenems, has also been found in a plasmid in clinical isolates.Citation37
Conjugative and mobilizable plasmids. To date, two conjugative plasmids have been identified: the B. fragilis 41kb pBF4 plasmid and B. ovatus 80.6kb pBI136 plasmid. DNA sequencing of the transfer regions of these plasmids has been limited due to A-T rich tracts that confound assembly and analyses. Only one gene, bctA, encoding a 110kD protein that localizes to the membrane, has been identified to be required in mating process.Citation38 Multiple mobilizable plasmids have been identified, that can only be transferred via the mating channel formed by other co-resided conjugative elements; these include B. fragilis pLV22a and pBFTM10.Citation39
Cryptic plasmids. Bacteroides spp are also known for harboring small molecular weight cryptic plasmids at high frequency (50%).Citation40 These small plasmids are characterized in size classes: class I, 2.7 kb, class IIA, 4.2 kb, class IIB, 5.0kb, class IIC, 7.9 kb and class III, 5.6 kb.Citation40,Citation41 The majority of small plasmids are found in class I, IIA and III. Class IIB and IIC are rare among both normal flora and clinical isolates.Citation40 These small molecular weight plasmids are called cryptic because, beside basic plasmid maintenance functions like replication, mobilization and in some cases, stability, they do not encode for any other obviously biological useful traits such as antibiotic resistance or production of virulence-associated proteins. For example, pBI143, a 2.7 kb plasmid, and pB8–51, a 4.2 kb plasmid, have been found to encode only replication and mobilization functions.Citation42–Citation44 pBF35, a representative of the most frequent class III plasmids, and originating in Hungary, was also found to encode only replication, mobilization and stability functions.Citation41
At first glance, it may appear that cryptic plasmids have no clinical importance because they do not carry resistance genes. However, they can have important clinical effects since they are capable of acquiring one or more antibiotic resistance genes and becoming mobile via integration with other conjugative or mobilizable transposons (or even other plasmids), resulting in further dissemination of antibiotic resistance genes. In addition, the abundance and diversity of these cryptic plasmids is cause for concern.
Transposons.
Transposons, both mobilizable and conjugative, are most often located on the bacterial genome, do not replicate independently, and are copied along with the chromosomal DNA.
Mobilizable transposons. Mobilizable transposons, like mobilizable plasmids, cannot transfer autonomously, but can be disseminated from donor bacteria via a co-resident “helper” element.Citation6 They use the mating apparatus of a co-resident conjugative element such as a conjugative plasmid or transposon for transfer to a recipient cell. Mobilizable transposons are invariably smaller than conjugative transposons and carry genes whose products are required for excision, DNA processing and integration of the element. However, they do not encode conjugal apparatus components and have to depend on co-resident conjugal elements like conjugative transposons. Some well-characterized mobilizable transposons in the bacteroidetes are the B. fragilis 9.6kb Tn4399,Citation45B. fragilis 4.69kb Tn5520,Citation46B. fragilis 15.3kb cLV25,Citation47B. uniformis 10.3kb NBU1, B. uniformis 11.1kb NBU248 and B. vulgatus 12.5kb Tn4555.49 Tn4399 requires the gene products encoded by mocA (a predicted relaxase MocA), and mocB, for mobilization.Citation50 During transposition, Tn4399 creates a 3-bp target site repeat and inserts an extra 5bp between the right inverted repeat and the target site repeat.Citation51 However, other known mobilizable transposons require just one gene, encoding a relaxase, for mobilization. These are Tn5520 (BmpHCitation46,Citation52), Tn4555 (MobACitation53), NBU1 (MobN1), and NBU2 (MobN2Citation48). To date, the BmpH protein, encoded by the smallest known mobilizable transposon Tn5520, is the best characterized B. fragilis relaxase.Citation52
Conjugative transposons. Conjugative transposons (CTn's) are frequently found in Bacteroides spp More than 80% of Bacteroides strains contain at least one conjugative transposon.Citation8 Conjugative transposons in Bacteroides are often called “tetracycline resistance factors,” and many of them can be stimulated to transfer via tetracycline exposure. They range in size from 52kb – 150kb, and include B. fragilis BTF-37 (37kb),Citation54B. thetaiotaomicron CTnDOT (65kb),Citation55B. thetaiotaomicron CTnERL (52kb),Citation56B. thetaiotaomicron TcrEmrDOT (70kb),Citation57B. thetaiotaomicron TcrEmr7853 (70kb),Citation58B. thetaiotaomicron CTnBST (100kb),Citation59B. thetaiotaomicron CTnGERM1 (75kb),Citation60B. vulgatus CTn341 (52kb),Citation61B. fragilis CTn86 (57kb)Citation62 Of these, CTnDOT (65kb) from B. thetaiotaomicron is the best described. CTn's have also been referred to as “Tet elements” since most, but not all, carry a tetracycline resistance gene (usually tetQ).Citation35,Citation63 Many CTns also carry the rteABC gene cluster, whose products are involved in the regulation of conjugal transfer.Citation64,Citation65rteA and rteB genes encode a tetracycline inducible twocomponent regulatory system, which controls rteC expression.Citation65rteC, in turn, controls expression of genes required for excision of transmissible elements. Transcription of tetQ, rteA and rteB is constitutive but translation of these genes is elevated during exposure to tetracycline due to a translational attenuation mechanism. As a result, very low (sub-inhibitory) levels of tetracycline or its analogs can markedly elevate conjugal transfer of Tet elements and other co-resident factors by 1,000-to 10,000-fold even upon brief exposure.Citation64,Citation66 It should be noted that this induction of transfer, while notable in some elements, occurs alongside the constitutive transfer events that are hallmarks of all these elements, and that have likely been occurring for millenia. Many B. fragilis conjugative transposons also carry erythromycin resistance genes such as ermF (cTnDOT),Citation67ermB (cTnBST)Citation68 or ermG (cTnGERM1).Citation60
Conjugative transposons are mainly responsible for the spread of tetracycline and erythromycin resistance in clinical isolates of Bacteroides spp.Citation8 They are not only responsible for the dissemination of the antibiotic resistance genes which they themselves carry, but also for the transfer of antibiotic resistance genes harbored by co-resident mobilizable elements, via stimulation of the excision and transfer of those mobile elements. RteA and RteB encoded within the central regulatory region of the CTnDOT/ERL family of conjugative transposons regulate the excision and mobilization of the NBU mobilizable plasmids.Citation69
To date, CTnDOT (65kb) from B. thetaiotaomicron is the best studied conjugative transposon. Since it was first recovered from a patient with a Bacteroides infection,Citation8 CTnDOT has served as a model to study the transfer mechanism of conjugative transposons in Bacteroides spp. It contains an excision region, a central regulatory region and a transfer region, and its excision, integration and regulation have been extensively studied. Excision is the first step in CTnDOT transfer from the chromosome, and results in the formation of a non-replicating circular intermediate.Citation7 An operon containing genes required for excision (orf2c, orf2d, and exc) was identified and is regulated at the transcriptional level by the tetracycline-inducible regulatory proteins RteA, RteB and RteC.Citation65,Citation70–Citation72 Integration is the final step when the CTnDOT intermediate recombines with the recipient bacterial genome. The integration reaction requires IntDOT, a CTnDOT-encoded protein that is a member of the tyrosine recombinase family, as well as an uncharacterized Bacteroides host factor.Citation70,Citation73,Citation74 Interestingly, and somewhat surprisingly, the excision proteins Orf2C, Orf2d and Exc also appear to be involved in integration.Citation70 However, little is known about the organization and regulation of the transfer genes responsible for the formation of the conjugation apparatus that allows the transfer of co-resident mobilizable elements. Expression of the transfer genes (traA to traQ) is activated directly by the excision proteins, and independently of RteC.Citation75
Chimeric transposons. Due to their ability to integrate into DNA, it should not be surprising to find chimeric transposons, i.e., a transposon integrated into another transposon. However, to date, there are only two chimeric transposons in which a CTn inserted into another CTn, have been reported, a CTn in gram-positive bacteria, the streptococcal Tn5253Citation76; and a CTn in gram negative bacteria, the Bacteroides CTn12256.Citation77 Although the 188kb CTn12256 was isolated from a clinical isolate in 1977, only recently has its chimeric nature been studied in more detail.Citation78 By cloning parts of CTn12256 into fosmids and performing a PCR walking survey, Wang, et al. found that the element was a chimera formed by the integration of a CTnDOT type element (CTnDOT2) into another CTn, CTn3Bf, that is most similar to a putative CTn found in B. fragilis YCH46.Citation78 It is interesting that although CTnDOT2 has 98% identity to CTnDOT, the transfer of the chimeric transposon CTn12256 is not dependent on tetracycline stimulation like CTnDOT and most other Bacteroides sp transposons. Because the traG homolog on CTnBf3, but not the traG of CTnDOT2, was essential for transfer, it was thought that CTnBf3 possibly dominant in the transfer of CTn12256. However, that is not the case because CTnBf3 cannot transfer independently of CTnDOT2 due to a large deletion near the integration site of CTnDOT2. This suggests that the unstudied Orf BF2884 encoded by this deleted region may present an important transfer or mobilization gene.
How are Mobile Elements Transfered Within and from Bacteroides spp?
The primary mechanism responsible for the dissemination of genetic elements in Bacteroides spp is conjugation, one of the most important mechanisms of horizontal gene transfer in prokaryotes. However, the molecular mechanism(s) of this process is poorly understood in Bacteroides spp.
Conjugation, a subtype of the bacterial Type IV secretion system (T4SS), is defined as the uni-directional transfer of a single-stranded DNA molecule from a bacterial donor cell to a recipient cell, in a process requiring cell-to-cell contact.Citation79 During conjugation, one copy of the DNA strand is transferred to, and replicated in, the recipient cell. The parent DNA is retained and replicated in the donor cell. Transferred DNA molecules, which can be either plasmids or transposons, are of two types: conjugative and mobilizable. As described above, conjugative plasmids and transposons are autonomously- or self-transmissible elements, encoding all components necessary for transfer. Mobilizable plasmids and transposons are non-self-transmissible elements. Their transfers require the assistance of a co-resident conjugative transfer element. Conjugative elements tend to be large (> 30kb), while mobilizable elements are small (< 15kb).Citation80
All transfer elements contain a cis-acting origin of transfer (oriT) sequence where transfer is initiated. oriTs are specific sequences, about 30–500bp in length, most often located adjacent to the transfer initiation genes known as mobilization (“Mob”) genes, and forming a compact mobilization region.Citation81 A common feature of the oriT is the presence of inverted repeats juxtaposed near a DNA sequence that is nicked during the transfer process.Citation82 The nick (nic) site, a short stretch of about 10 nucleotides, is the site for recognition by the relaxase, required for the DNA transfer process.
Conjugation involves two major sets of events: Initiation (DNA processing) and conjugal apparatus formation as described below, and diagrammed in .
Initiation (DNA processing).
DNA processing includes binding, nicking and unwinding of the DNA, and these reactions are independent of conjugal apparatus formation.Citation81,Citation83 Processing occurs via the relaxosome, a nucleoprotein complex composed of specific proteins (mobilization proteins), one of which is the relaxase.Citation84,Citation85 This critical mobilization protein nicks the DNA to be transferred in a site- and strand- specific manner at the origin of transfer (oriT),Citation81,Citation83 and then covalently associates with the 5′-end of the nicked DNA via a phosphotyrosyl linkage. The nicked DNA is further unwound from the parent molecule, and transmitted in single-stranded fashion with 5′-3′ polarity from the donor to the recipient.Citation81 Single-stranded copies in both the donor and the recipient are then re-circularized and restored to the double-stranded form.Citation81 The passage from the donor to the recipient occurs through a specialized membrane-traversing channel called the conjugal apparatus (discussed below).
Relaxase proteins, the major mobilization proteins of the relaxosomes, are usually multifunctional, and thus contain two or more protein domains. The nicking domain is always located at the N-terminus of the protein.Citation80 At the C-terminus, a DNA helicase, DNA primase or other domain of unknown function is almost always found.Citation80 Crystal structures of some relaxases have been obtained, including that of the F plasmid TraI nicking domain, with and without a bound DNA substrate,Citation86,Citation87 the nicking domain of TrwC from plasmid R388 with bound DNACitation88,Citation89 and that of MobA from Inc.Q plasmid R1162.Citation90 In many cases, the nicking domain itself contains at least three conserved protein motifs. Motif I contains the active site tyrosine, which creates a single-stranded 5′ DNA nick through a trans-esterification reaction similar to that of type I topoisomerase.Citation83 This reaction involves the nucleophilic attack on the DNA-phosphate backbone by the hydroxyl group of the tyrosine, resulting in a reversible covalent phosphodiester bond.Citation81,Citation91 Motif II is likely responsible for the recognition and noncovalent binding of the relaxase with the end of the trailing region 3′ to the nic site. Motif III is histidine-rich and is called HUH (His-hyrophobic residue-His) or HHH (His-His-His). This motif likely facilitates the cleavage reaction (trans-esterification) by abstracting a proton from the terminal tyrosine hydroxyl, allowing the oxygen moiety to act as a nucleophile.Citation81 The termination of strand-transfer occurs via a second cleavage reaction, releasing a single-stranded DNA molecule in the recipient cell.Citation81
Most relaxases require the activity of accessory proteins to alter the conformation of the DNA to facilitate relaxase binding. The E. coli plasmid RP4-encoded relaxosome requires three proteins (two cognate proteins and the host encoded integration host factor, IHF) to achieve the conformation required for the relaxase to bind and nick the DNA.Citation91 Similarly, relaxase activity of the TrwC relaxase of the R388 plasmid system also requires assistance from TrwA.Citation92 In the F plasmid system, TraY enhances the relaxase/helicase activity of TraI.Citation93 In Bacteroides spp, to date, the mobilization region of CTn341Citation94 and Tn5520Citation52 have been elaborated in detail. In CTn341 system, activity of the relaxase mobB required the accessory protein mobA.Citation94 However, in Tn5520, the relaxase BmpH is the first relaxase in Bacteroides spp reported to not require any accessory protein.Citation52
Conjugation apparatus formation.
The second major process in conjugation is the formation of the conjugal- or mating apparatus. The conjugal apparatus (CA) is a multi-protein channel that is assembled across donor and recipient cell membranes during conjugation, through which the DNA strand is transferred.Citation95,Citation96 A pilus or other surface filament or proteins(s) may also be produced to facilitate adhesion and contact between two cells.Citation97,Citation98
Although the formation of the conjugal apparatus has been well studied in Agrobacterium tumefaciens Ti plasmids and E.coli F, RP4 and R388 plasmids, little is known about its structure and function in Bacteroides spp In E. coli and A. tumefaciens, this membrane channel is formed by 10–12 proteins ().Citation95,Citation99–Citation101 In the E. coli RP4 plasmid system, the mating channel is composed of 10 mating-pair gene products, a TraF pilin support protein and the coupling protein TraG.Citation95,Citation102 In E. coli F plasmid system, the channel is composed of 11 proteins including the coupling protein TraD.Citation103 In A. tumefaciens, each of 12 proteins named VirB1 to VirB11 and VirD4 has been extensively characterized.Citation104,Citation105 Recently, a cryo-electron microscopic structure of the core complex of the conjugal apparatus encoded by the E. coli conjugative plasmid pKM101 showed that the CA complex is 108Å wide and spanned the inner and outer membranes.Citation106 However, in Bacteroides spp the nature and function of the CA in even the best studied elements is still poorly understood. To date, the only detailed description of CA-encoding genes in Bacteroides spp is from a study of the B. vulgatus CTn341 element, where the requirement of each CA gene was assessed via the generation of gene mutants.Citation61
Specificity (coupling protein).
One of the CA's most important components is the coupling protein (CP). CPs appear to be encoded by all conjugative plasmids and transposons, but not by mobilizable elements. The CP is unique to conjugation and is considered to be the first point of contact that the relaxosome and/or tDNA makes with the CA. The best characterized CPs are TrwB of plasmid R388 (Inc.W group), TraD of F plasmids (Inc.F group), TraG of RP4 plasmids (Inc.P group), and VirD4 of A. tumefaciens Ti plasmids.Citation107 These proteins share the following characteristics: (1) they are composed of transmembrane a-helices in their N-terminal region that mediate anchoring to the inner membrane. Indeed, they are integral inner membrane proteins.Citation108–Citation110 They also typically have a cytoplasmic C-terminal domain.Citation95,Citation108,Citation111,Citation112 Thus, their location is the link between a cytoplasmic system and the membrane complex. (2) CPs have a nucleotide binding motif, and can bind both single- and double- stranded DNA, suggesting a specific role in DNA transfer.Citation109 (3) A CP has a Walker box domain, and cytoplasmic domains that interact with the relaxosome.Citation113 The presence of Walker box motifs suggest that they use ATP hydrolysis as an energy source. It is speculated that when CP interacts with the relaxosome, the Walker-box mediates ATP hydrolysis to provide energy to “pump” the relaxosome through the CA and into the recipient cell.Citation114,Citation115 With this role, CPs are considered to be “gatekeepers” of conjugation. The A. tumefaciens CA has two of these CP “gatekeepers,” VirD4 and its required partner VirB4, both of which have nucleotide binding activity.Citation113,Citation116,Citation117 However, in E. coli, one CP has been described for each conjugative system. (4) CPs are often multimeric proteins. The crystal structure of the soluble cytoplasmic domain of TrwB, the CP of the Inc.W plasmid R388, shows that it is a hexameric protein, resembles a ring helicase.Citation118
Most importantly, and in many transfer systems, including those elaborated by the E. coli F plasmids and A. tumefaciens Ti plasmids, CPs are highly selective for the cognate relaxosome.Citation119,Citation120 Thus, a CA will only allow transfer of the plasmid that encodes it, as well as co-resident plasmids belonging to the same incompatibility group. All other plasmids and mobile elements are strictly excluded. However, and interestingly, in B. fragilis, putative CPs do not appear to harbor this selectivity. Conjugative transposons facilitate their own transfer, as well as that of all co-resident mobile elements, and “incompatibility” does not seem to play any role in the selection of molecules destined for dissemination. Thus, mobile elements can be transferred within, and from B. fragilis even to bacteria from other genera. This promiscuous transfer may be one significant driving force behind Bacteroides spp as reservoirs of transferable antibiotic-resistance conferring elements.
Combating Antibiotic Resistance by Targeting Bacterial Conjugation
With the alarming rise and spread of antibiotic resistance to even new generations of antibiotics, the need to prevent the dissemination of antibiotic resistance is more urgent than ever before. Obviously, it is biologically impractical to use approaches that will eradicate all Bacteroides spp organisms, antibiotic-resistant or not. Since conjugation is the primary means by which Bacteroides spp. disseminate antibiotic resistance (and other) genes, interventions that target this process present an attractive approach to preventing unwanted horizontal DNA transfer - and only in the subset of organisms that are competent to do so. Currently, multiple groups are exploring non-antibiotic-based methodologies to prevent conjugation-based DNA transfer.Citation15,Citation121–Citation124 Different methodologies to inhibit the conjugative relaxase have been tested. Antibody libraries against the TrwC relaxase of conjugative plasmid R388 have been used to block its nicking activity within recipient cells.Citation122 Other studies report being able to disrupt the conjugation process by using specific inhibitors to the F plasmid relaxase.Citation121 Interestingly, a short palindromic repeat (CRISPR) focusing on RNA interference can also limit gene transfer - this has been tested in staphylococci by targeting relaxase genes.Citation123 Thus, one approach in the search for interventions to mitigate antibiotic resistance gene spread will likely focus on conjugation system-based targets.
Conclusion
Bacteroides spp, the most prominent group of bacteria in the gut, can be both beneficial symbionts, as well as dangerous opportunistic pathogens. Arguably, one of the most significant traits that characterizes the Bacteroides as important clinical pathogens is their capacity to harbor a plethora of transmissible genetic elements carrying many antibiotic resistance genes. Since these genes are efficiently disseminated and acquired, Bacteroides spp are now considered to be reservoirs of these antibiotic resistance traits. Despite some of the advances described in this review, it should be emphasized that the majority of the mechanistic aspects of conjugative transfer—the pre-eminent process that drives the spread of antibiotic resistance in the genus—still remain to be elucidated. In-depth studies on Bacteroides spp conjugational components and mechanisms will thus provide insights to designing effective interventions that might focus on conjugation-system targets to prevent antibiotic resistance gene transfer.
Abbreviations
| CA | = | conjugation apparatus |
| CRISPR | = | Clustered Regularly Interspaced Short Palindromic Repeats |
| CTn | = | conjugative transposon |
| ETBF | = | enterotoxigenic Bacteroides fragilis |
| IBD | = | inflammatory bowel disease |
| IHF | = | Integrative Host Factor |
| MIC | = | minimum inhibitory concentration |
| MLS | = | macrolide-lincosamide-streptogramin |
| Mob | = | mobilization |
| oriT | = | origin of transfer |
Figures and Tables
Figure 1 Schematic highlighting the differences between conjugative and mobilizable elements. In the former, both DNA processing as well as mating apparatus functions are encoded, whereas, in the latter, only DNA-processing functions are elaborated.
Figure 2 Schematic representing the major events occurring during conjugative DNA transfer. Cell membranes separating donor and recipient bacteria are depicted as solid black lines. The transferring element (plasmid-like in this example) is shown harboring an origin of conjugative transfer (oriT) as well as a mobilization (MOB) protein-encoding segment (Mob genes). As described in the text, a single-stranded DNA molecule is generated during the conjugation process, and translocated to the mating apparatus, of which one member is the coupling protein.
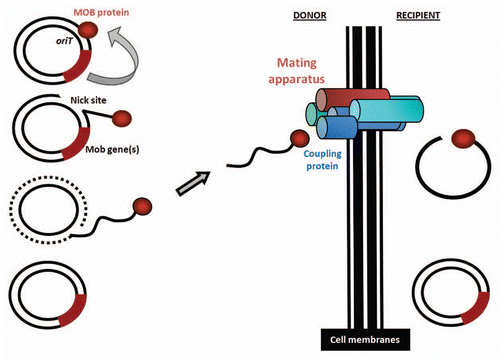
Table 1 Mobile genetic elements found in Bacteroides spp.
Table 2 Comparison of conjugation apparatus components of A. tumefacien, E. coli and Bacteroides spp. mating systems.
Acknowledgments
Research in the GV laboratory is supported by grants from the US Department of Veterans Affairs and the USDA-CSREES Hatch Program.
References
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124:837 - 848; PMID: 16497592; http://dx.doi.org/10.1016/j.cell.2006.02.017
- Xu J, Chiang HC, Bjursell MK, Gordon JI. Message from a human gut symbiont: sensitivity is a prerequisite for sharing. Trends Microbiol 2004; 12:21 - 28; PMID: 14700548; http://dx.doi.org/10.1016/j.tim.2003.11.007
- Mai V, Morris JG Jr. Colonic bacterial flora: changing understandings in the molecular age. J Nutr 2004; 134:459 - 464; PMID: 14747689
- Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003; 361:512 - 519; PMID: 12583961; http://dx.doi.org/10.1016/S0140-6736(03)12489-0
- Salyers AA. Bacteroides of the human lower intestinal tract. Annu Rev Microbiol 1984; 38:293 - 313; PMID: 6388494; http://dx.doi.org/10.1146/annurev.mi.38.100184.001453
- Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 2007; 20:593 - 621; PMID: 17934076; http://dx.doi.org/10.1128/CMR.00008-07
- Whittle G, Shoemaker NB, Salyers AA. The role of Bacteroides conjugative transposons in the dissemination of antibiotic resistance genes. Cell Mol Life Sci 2002; 59:2044 - 2054; PMID: 12568330; http://dx.doi.org/10.1007/s000180200004
- Shoemaker NB, Vlamakis H, Hayes K, Salyers AA. Evidence for extensive resistance gene transfer among Bacteroides spp. and among Bacteroides and other genera in the human colon. Appl Environ Microbiol 2001; 67:561 - 568; PMID: 11157217; http://dx.doi.org/10.1128/AEM.67.2.561-568.2001
- Reid G. When microbe meets human. Clin Infect Dis 2004; 39:827 - 830; PMID: 15472815; http://dx.doi.org/10.1086/423387
- Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 2001; 414:555 - 558; PMID: 11734857; http://dx.doi.org/10.1038/35107092
- Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science 2005; 307:1778 - 1781; PMID: 15774760; http://dx.doi.org/10.1126/science.1106469
- Baughn AD, Malamy MH. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 2004; 427:441 - 444; PMID: 14749831; http://dx.doi.org/10.1038/nature02285
- Waters VL. Conjugative transfer in the dissemination of beta-lactam and aminoglycoside resistance. Front Biosci 1999; 4:D433 - D456; PMID: 10228095; http://dx.doi.org/10.2741/Waters
- Brook I. Endocarditis due to anaerobic bacteria. Cardiology 2002; 98:1 - 5; PMID: 12373039; http://dx.doi.org/10.1159/000064684
- Vedantam G. Antimicrobial resistance in Bacteroides spp.: occurrence and dissemination. Future Microbiol 2009; 4:413 - 423; PMID: 19416011; http://dx.doi.org/10.2217/fmb.09.12
- Shinagawa N, Osanai H, Hirata K, Furuhata T, Mizukuchi T, Yanai Y, et al. Bacteria isolated from surgical infections and its susceptibilities to antimicrobial agents-special references to bacteria isolated between April 2009 and March 2010. Jpn J Antibiot 2011; 64:125 - 169; PMID: 21861307
- Papaparaskevas J, Katsandri A, Pantazatou A, Stefanou I, Avlamis A, Legakis NJ, et al. Epidemiological characteristics of infections caused by Bacteroides, Prevotella and Fusobacterium species: a prospective observational study. Anaerobe 2011; 17:113 - 117; PMID: 21664284; http://dx.doi.org/10.1016/j.anaerobe.2011.05.013
- Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease?. Dig Dis Sci 2004; 49:1425 - 1432; PMID: 15481314; http://dx.doi.org/10.1023/B:DDAS.0000042241.13489.88
- Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J Jr. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis 2000; 6:171 - 174; PMID: 10756151; http://dx.doi.org/10.3201/eid0602.000210
- Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect 2006; 12:782 - 786; PMID: 16842574
- Yoshino Y, Kitazawa T, Ikeda M, Tatsuno K, Yanagimoto S, Okugawa S, et al. Clinical features of Bacteroides bacteremia and their association with colorectal carcinoma. Infection 2011; In press PMID: 21773761; http://dx.doi.org/10.1007/s15010-011-0159-8
- Polk BF, Kasper DL. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med 1977; 86:569 - 571; PMID: 322563
- Koeth LM, Good CE, Appelbaum PC, Goldstein EJ, Rodloff AC, Claros M, et al. Surveillance of susceptibility patterns in 1297 European and US anaerobic and capnophilic isolates to co-amoxiclav and five other antimicrobial agents. J Antimicrob Chemother 2004; 53:1039 - 1044; PMID: 15128729; http://dx.doi.org/10.1093/jac/dkh248
- Snydman DR, Jacobus NV, McDermott LA, Ruthazer R, Golan Y, Goldstein EJ, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother 2007; 51:1649 - 1655; PMID: 17283189; http://dx.doi.org/10.1128/AAC.01435-06
- Betriu C, Culebras E, Gomez M, Lopez F, Rodriguez-Avial I, Picazo JJ. Resistance trends of the Bacteroides fragilis group over a 10-year period, 1997 to 2006, in Madrid, Spain. Antimicrob Agents Chemother 2008; 52:2686 - 2690; PMID: 18474575; http://dx.doi.org/10.1128/AAC.00081-08
- Hedberg M, Nord CE. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe. Clin Microbiol Infect 2003; 9:475 - 488; PMID: 12848722; http://dx.doi.org/10.1046/j.1469-0691.2003.00674.x
- Stein GE, Goldstein EJ. Fluoroquinolones and anaerobes. Clin Infect Dis 2006; 42:1598 - 1607; PMID: 16652318; http://dx.doi.org/10.1086/503907
- Betriu C, Rodriguez-Avial I, Gomez M, Culebras E, Picazo JJ. Changing patterns of fluoroquinolone resistance among Bacteroides fragilis group organisms over a 6-year period (1997–2002). Diagn Microbiol Infect Dis 2005; 53:221 - 223; PMID: 16243476; http://dx.doi.org/10.1016/j.diagmicrobio.2005.06.012
- Vieira BD, Boente JM, Rodrigues RF, Miranda K, Avelar KE, R MCPD, Candida de SFM. Decreased susceptibility to nitroimidazoles among Bacteroides species in Brazil. Curr Microbiol 2006; 52:27 - 32; PMID: 16391998
- Chaudhry R, Mathur P, Dhawan B, Kumar L. Emergence of metronidazole-resistant Bacteroides fragilis, India. Emerg Infect Dis 2001; 7:485 - 486; PMID: 11384542
- Schapiro JM, Gupta R, Stefansson E, Fang FC, Limaye AP. Isolation of metronidazole-resistant Bacteroides fragilis carrying the nimA nitroreductase gene from a patient in Washington State. J Clin Microbiol 2004; 42:4127 - 4129; PMID: 15364999; http://dx.doi.org/10.1128/OCM.42.9.4127-4129.2004
- Nagy E, Soki J, Urban E, Szoke I, Fodor E, Edwards R. Occurrence of metronidazole and imipenem resistance among Bacteroides fragilis group clinical isolates in Hungary. Acta Biol Hung 2001; 52:271 - 280; PMID: 11426861; http://dx.doi.org/10.1556/ABiol.52.2001.2-3.11
- Wójcik-Stojek B, Bulanda M, Martirosian G, Heczko P, Meisel-Mikolajczyk F. In vitro antibiotic susceptibility of Bacteroides fragilis strains isolated from excised appendix of patients with phlegmonous or gangrenous appendicitis. Acta Microbiol Pol 2000; 49:171 - 175; PMID: 11093680
- Dubreuil L, Odou MF. Anaerobic bacteria and antibiotics: What kind of unexpected resistance could I find in my laboratory tomorrow?. Anaerobe 2010; 16:555 - 559; PMID: 20971200; http://dx.doi.org/10.1016/j.anaerobe.2010.10.002
- Salyers AA, Shoemaker NB, Stevens AM, Li LY. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol Rev 1995; 59:579 - 590; PMID: 8531886
- Löfmark S, Fang H, Hedberg M, Edlund C. Inducible metronidazole resistance and nim genes in clinical Bacteroides fragilis group isolates. Antimicrob Agents Chemother 2005; 49:1253 - 1256; PMID: 15728943; http://dx.doi.org/10.1128/AAC.49.3.1253-1256.2005
- Nakano V, Padilla G, do Valle Marques M, Avila-Campos MJ. Plasmid-related beta-lactamase production in Bacteroides fragilis strains. Res Microbiol 2004; 155:843 - 846; PMID: 15567279; http://dx.doi.org/10.1016/j.resmic.2004.06.011
- Morgan RM, Macrina FL. bctA: a novel pBF4 gene necessary for conjugal transfer in Bacteroides spp. Microbiology 1997; 143:2155 - 2165; PMID: 9245805; http://dx.doi.org/10.1099/00221287-143-7-2155
- Smith CJ, Tribble GD, Bayley DP. Genetic elements of Bacteroides species: a moving story. Plasmid 1998; 40:12 - 29; PMID: 9657930; http://dx.doi.org/10.1006/plas.1998.1347
- Callihan DR, Young FE, Clark VL. Identification of three homology classes of small, cryptic plasmids in intestinal Bacteroides species. Plasmid 1983; 9:17 - 30; PMID: 6300942; http://dx.doi.org/10.1016/0147-619X(83)90028-8
- Sóki J, Wareham DW, Ratkai C, Aduse-Opoku J, Urban E, Nagy E. Prevalence, nucleotide sequence and expression studies of two proteins of a 5.6kb, class III, Bacteroides plasmid frequently found in clinical isolates from European countries. Plasmid 2010; 63:86 - 97; PMID: 20026106; http://dx.doi.org/10.1016/j.plasmid.2009.12.002
- Smith CJ, Rollins LA, Parker AC. Nucleotide sequence determination and genetic analysis of the Bacteroides plasmid, pBI143. Plasmid 1995; 34:211 - 222; PMID: 8825374; http://dx.doi.org/10.1006/plas.1995.0007
- Valentine PJ, Shoemaker NB, Salyers AA. Mobilization of Bacteroides plasmids by Bacteroides conjugal elements. J Bacteriol 1988; 170:1319 - 1324; PMID: 3343220
- Novicki TJ, Hecht DW. Characterization and DNA sequence of the mobilization region of pLV22a from Bacteroides fragilis. J Bacteriol 1995; 177:4466 - 4473; PMID: 7635830
- Hecht DW, Malamy MH. Tn4399, a conjugal mobilizing transposon of Bacteroides fragilis. J Bacteriol 1989; 171:3603 - 3608; PMID: 2544548
- Vedantam G, Novicki TJ, Hecht DW. Bacteroides fragilis transfer factor Tn5520: the smallest bacterial mobilizable transposon containing single integrase and mobilization genes that function in Escherichia coli. J Bacteriol 1999; 181:2564 - 2571; PMID: 10198023
- Bass KA, Hecht DW. Isolation and characterization of cLV25, a Bacteroides fragilis chromosomal transfer factor resembling multiple Bacteroides sp. mobilizable transposons. J Bacteriol 2002; 184:1895 - 1904; PMID: 11889096; http://dx.doi.org/10.1128/JB.184.7.1895-1904.2002
- Li LY, Shoemaker NB, Wang GR, Cole SP, Hashimoto MK, Wang J, et al. The mobilization regions of two integrated Bacteroides elements, NBU1 and NBU2, have only a single mobilization protein and may be on a cassette. J Bacteriol 1995; 177:3940 - 3945; PMID: 7608064
- Smith CJ, Parker AC. Identification of a circular intermediate in the transfer and transposition of Tn4555, a mobilizable transposon from Bacteroides spp. J Bacteriol 1993; 175:2682 - 2691; PMID: 8386723
- Murphy CG, Malamy MH. Characterization of a “mobilization cassette” in transposon Tn4399 from Bacteroides fragilis. J Bacteriol 1993; 175:5814 - 5823; PMID: 8397185
- Hecht DW, Thompson JS, Malamy MH. Characterization of the termini and transposition products of Tn4399, a conjugal mobilizing transposon of Bacteroides fragilis. Proc Natl Acad Sci USA 1989; 86:5340 - 5344; PMID: 2546154; http://dx.doi.org/10.1073/pnas.86.14.5340
- Vedantam G, Knopf S, Hecht DW. Bacteroides fragilis mobilizable transposon Tn5520 requires a 71 base pair origin of transfer sequence and a single mobilization protein for relaxosome formation during conjugation. Mol Microbiol 2006; 59:288 - 300; PMID: 16359335; http://dx.doi.org/10.1111/j.1365-2958.2005.04934.x
- Smith CJ, Parker AC. A gene product related to Tral is required for the mobilization of Bacteroides mobilizable transposons and plasmids. Mol Microbiol 1996; 20:741 - 750; PMID: 8793871; http://dx.doi.org/10.1111/j.1365-2958.1996.tb02513.x
- Vedantam G, Hecht DW. Isolation and characterization of BTF-37: chromosomal DNA captured from Bacteroides fragilis that confers self-transferability and expresses a pilus-like structure in Bacteroides spp. and Escherichia coli. J Bacteriol 2002; 184:728 - 738; PMID: 11790742; http://dx.doi.org/10.1128/JB.184.3.728-738.2002
- Bonheyo G, Graham D, Shoemaker NB, Salyers AA. Transfer region of a bacteroides conjugative transposon, CTnDOT. Plasmid 2001; 45:41 - 51; PMID: 11319931; http://dx.doi.org/10.1006/plas.2000.1495
- Bonheyo GT, Hund BD, Shoemaker NB, Salyers AA. Transfer region of a Bacteroides conjugative transposon contains regulatory as well as structural genes. Plasmid 2001; 46:202 - 209; PMID: 11735369; http://dx.doi.org/10.1006/plas.2001.1545
- Li LY, Shoemaker NB, Salyers AA. Location and characteristics of the transfer region of a Bacteroides conjugative transposon and regulation of transfer genes. J Bacteriol 1995; 177:4992 - 4999; PMID: 7665476
- Nikolich MP, Shoemaker NB, Wang GR, Salyers AA. Characterization of a new type of Bacteroides conjugative transposon, Tcr Emr 7853. J Bacteriol 1994; 176:6606 - 6612; PMID: 7961412
- Gupta A, Vlamakis H, Shoemaker N, Salyers AA. A new Bacteroides conjugative transposon that carries an ermB gene. Appl Environ Microbiol 2003; 69:6455 - 6463; PMID: 14602600; http://dx.doi.org/10.1128/AEM.69.11.6455-6463.2003
- Wang Y, Wang GR, Shelby A, Shoemaker NB, Salyers AA. A newly discovered Bacteroides conjugative transposon, CTnGERM1, contains genes also found in gram-positive bacteria. Appl Environ Microbiol 2003; 69:4595 - 4603; PMID: 12902247; http://dx.doi.org/10.1128/AEM.69.8.4595-4603.2003
- Bacic M, Parker AC, Stagg J, Whitley HP, Wells WG, Jacob LA, et al. Genetic and structural analysis of the Bacteroides conjugative transposon CTn341. J Bacteriol 2005; 187:2858 - 2869; PMID: 15805532; http://dx.doi.org/10.1128/JB.187.8.2858-2869.2005
- Buckwold SL, Shoemaker NB, Sears CL, Franco AA. Identification and characterization of conjugative transposons CTn86 and CTn9343 in Bacteroides fragilis strains. Appl Environ Microbiol 2007; 73:53 - 63; PMID: 17071793; http://dx.doi.org/10.1128/AEM.01669-06
- Shoemaker NB, Barber RD, Salyers AA. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol 1989; 171:1294 - 1302; PMID: 2646276
- Shoemaker NB, Salyers AA. Tetracycline-dependent appearance of plasmidlike forms in Bacteroides uniformis 0061 mediated by conjugal Bacteroides tetracycline resistance elements. J Bacteriol 1988; 170:1651 - 1657; PMID: 2832373
- Stevens AM, Shoemaker NB, Li LY, Salyers AA. Tetracycline regulation of genes on Bacteroides conjugative transposons. J Bacteriol 1993; 175:6134 - 6141; PMID: 8407786
- Rashtchian A, Dubes GR, Booth SJ. Tetracycline-inducible transfer of tetracycline resistance in Bacteroides fragilis in the absence of detectable plasmid DNA. J Bacteriol 1982; 150:141 - 147; PMID: 7061390
- Whittle G, Hund BD, Shoemaker NB, Salyers AA. Characterization of the 13-kilobase ermF region of the Bacteroides conjugative transposon CTnDOT. Appl Environ Microbiol 2001; 67:3488 - 3495; PMID: 11472924; http://dx.doi.org/10.1128/AEM.67.8.3488-3495.2001
- Wesslund NA, Wang GR, Song B, Shoemaker NB, Salyers AA. Integration and excision of a newly discovered bacteroides conjugative transposon, CTnBST. J Bacteriol 2007; 189:1072 - 1082; PMID: 17122349; http://dx.doi.org/10.1128/JB.01064-06
- Stevens AM, Sanders JM, Shoemaker NB, Salyers AA. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol 1992; 174:2935 - 2942; PMID: 1569023
- Cheng Q, Sutanto Y, Shoemaker NB, Gardner JF, Salyers AA. Identification of genes required for excision of CTnDOT, a Bacteroides conjugative transposon. Mol Microbiol 2001; 41:625 - 632; PMID: 11532130; http://dx.doi.org/10.1046/j.1365-2958.2001.02519.x
- Park J, Salyers AA. Characterization of the Bacteroides CTnDOT regulatory protein RteC. J Bacteriol 2011; 193:91 - 97; PMID: 21037014; http://dx.doi.org/10.1128/JB.01015-10
- Moon K, Shoemaker NB, Gardner JF, Salyers AA. Regulation of excision genes of the Bacteroides conjugative transposon CTnDOT. J Bacteriol 2005; 187:5732 - 5741; PMID: 16077120; http://dx.doi.org/10.1128/JB.187.16.5732-5741.2005
- Malanowska K, Salyers AA, Gardner JF. Characterization of a conjugative transposon integrase, IntDOT. Mol Microbiol 2006; 60:1228 - 1240; PMID: 16689798; http://dx.doi.org/10.1111/j.1365-2958.2006.05164.x
- Wood MM, Dichiara JM, Yoneji S, Gardner JF. CTnDOT integrase interactions with attachment site DNA and control of directionality of the recombination reaction. J Bacteriol 2010; 192:3934 - 3943; PMID: 20511494; http://dx.doi.org/10.1128/JB.00351-10
- Jeters RT, Wang GR, Moon K, Shoemaker NB, Salyers AA. Tetracycline-associated transcriptional regulation of transfer genes of the Bacteroides conjugative transposon CTnDOT. J Bacteriol 2009; 191:6374 - 6382; PMID: 19700528; http://dx.doi.org/10.1128/JB.00739-09
- Ayoubi P, Kilic AO, Vijayakumar MN. Tn5253, the pneumococcal omega (cat tet) BM6001 element, is a composite structure of two conjugative transposons, Tn5251 and Tn5252. J Bacteriol 1991; 173:1617 - 1622; PMID: 1847905
- Bedzyk LA, Shoemaker NB, Young KE, Salyers AA. Insertion and excision of Bacteroides conjugative chromosomal elements. J Bacteriol 1992; 174:166 - 172; PMID: 1309516
- Wang GR SN, Jeters RT, Salyers AA. CTn12256, a chimeric Bacteroides conjugative transposon that consists of two independently active mobile elements. Plasmid 2011; 66:93 - 105; PMID: 21777612; http://dx.doi.org/10.1016/j.plasmid.2011.06.003
- Willetts N, Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev 1984; 48:24 - 41; PMID: 6201705
- Garcillán-Barcia MP, Francia MV, de la Cruz F. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol Rev 2009; 33:657 - 687; PMID: 19396961; http://dx.doi.org/10.1111/j.1574-6976.2009.00168.x
- Lanka E, Wilkins BM. DNA processing reactions in bacterial conjugation. Annu Rev Biochem 1995; 64:141 - 169; PMID: 7574478; http://dx.doi.org/10.1146/annurev.bi.64.070195.001041
- Pansegrau W, Balzer D, Kruft V, Lurz R, Lanka E. In vitro assembly of relaxosomes at the transfer origin of plasmid RP4. Proc Natl Acad Sci USA 1990; 87:6555 - 6559; PMID: 2168553; http://dx.doi.org/10.1073/pnas.87.17.6555
- Pansegrau W, Schroder W, Lanka E. Relaxase (TraI) of IncP alpha plasmid RP4 catalyzes a site-specific cleaving-joining reaction of single-stranded DNA. Proc Natl Acad Sci USA 1993; 90:2925 - 2929; PMID: 8385350; http://dx.doi.org/10.1073/pnas.90.7.2925
- Pansegrau W, Ziegelin G, Lanka E. The origin of conjugative IncP plasmid transfer: interaction with plasmid-encoded products and the nucleotide sequence at the relaxation site. Biochim Biophys Acta 1988; 951:365 - 374; PMID: 2850014
- Pansegrau W, Lanka E. Mechanisms of initiation and termination reactions in conjugative DNA processing. Independence of tight substrate binding and catalytic activity of relaxase (TraI) of IncPalpha plasmid RP4. J Biol Chem 1996; 271:13068 - 13076; PMID: 8662726; http://dx.doi.org/10.1074/jbc.271.22.13068
- Larkin C, Datta S, Nezami A, Dohm JA, Schildbach JF. Crystallization and preliminary X-ray characterization of the relaxase domain of F factor TraI. Acta Crystallogr D Biol Crystallogr 2003; 59:1514 - 1516; PMID: 12876370; http://dx.doi.org/10.1107/S0907444903012964
- Larkin C, Haft RJ, Harley MJ, Traxler B, Schildbach JF. Roles of active site residues and the HUH motif of the F plasmid TraI relaxase. J Biol Chem 2007; 282:33707 - 33713; PMID: 17890221; http://dx.doi.org/10.1074/jbc.M703210200
- Boer R, Russi S, Guasch A, Lucas M, Blanco AG, Perez-Luque R, et al. Unveiling the molecular mechanism of a conjugative relaxase: The structure of TrwC complexed with a 27-mer DNA comprising the recognition hairpin and the cleavage site. J Mol Biol 2006; 358:857 - 869; PMID: 16540117; http://dx.doi.org/10.1016/j.jmb.2006.02.018
- Guasch A, Lucas M, Moncalian G, Cabezas M, Perez-Luque R, Gomis-Ruth FX, et al. Recognition and processing of the origin of transfer DNA by conjugative relaxase TrwC. Nat Struct Biol 2003; 10:1002 - 1010; PMID: 14625590; http://dx.doi.org/10.1038/nsb1017
- Monzingo AF, Ozburn A, Xia S, Meyer RJ, Robertus JD. The structure of the minimal relaxase domain of MobA at 2.1 A resolution. J Mol Biol 2007; 366:165 - 178; PMID: 17157875; http://dx.doi.org/10.1016/j.jmb.2006.11.031
- Byrd DR, Matson SW. Nicking by transesterification: the reaction catalysed by a relaxase. Mol Microbiol 1997; 25:1011 - 1022; PMID: 9350859; http://dx.doi.org/10.1046/j.1365-2958.1997.5241885.x
- Moncalián G, de la Cruz F. DNA binding properties of protein TrwA, a possible structural variant of the Arc repressor superfamily. Biochim Biophys Acta 2004; 1701:15 - 23; PMID: 15450172
- Lum PL, Rodgers ME, Schildbach JF. TraY DNA recognition of its two F factor binding sites. J Mol Biol 2002; 321:563 - 578; PMID: 12206773; http://dx.doi.org/10.1016/S0022-2836(02)00680-0
- Peed L, Parker AC, Smith CJ. Genetic and functional analyses of the mob operon on conjugative transposon CTn341 from Bacteroides spp. J Bacteriol 2010; 192:4643 - 4650; PMID: 20639338; http://dx.doi.org/10.1128/JB.00317-10
- Grahn AM, Haase J, Bamford DH, Lanka E. Components of the RP4 conjugative transfer apparatus form an envelope structure bridging inner and outer membranes of donor cells: implications for related macromolecule transport systems. J Bacteriol 2000; 182:1564 - 1574; PMID: 10692361; http://dx.doi.org/10.1128/JB.182.6.1564-1574.2000
- Samuels AL, Lanka E, Davies JE. Conjugative junctions in RP4-mediated mating of Escherichia coli. J Bacteriol 2000; 182:2709 - 2715; PMID: 10781537; http://dx.doi.org/10.1128/JB.182.10.2709-2715.2000
- Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu Rev Microbiol 2005; 59:451 - 485; PMID: 16153176; http://dx.doi.org/10.1146/annurev.micro.58.030603.123630
- Collins RF, Frye SA, Balasingham S, Ford RC, Tonjum T, Derrick JP. Interaction with type IV pili induces structural changes in the bacterial outer membrane secretin PilQ. J Biol Chem 2005; 280:18923 - 18930; PMID: 15753075; http://dx.doi.org/10.1074/jbc.M411603200
- Anthony KG, Klimke WA, Manchak J, Frost LS. Comparison of proteins involved in pilus synthesis and mating pair stabilization from the related plasmids F and R100-1: insights into the mechanism of conjugation. J Bacteriol 1999; 181:5149 - 5159; PMID: 10464182
- Li PL, Everhart DM, Farrand SK. Genetic and sequence analysis of the pTiC58 trb locus, encoding a matingpair formation system related to members of the type IV secretion family. J Bacteriol 1998; 180:6164 - 6172; PMID: 9829924
- Li PL, Hwang I, Miyagi H, True H, Farrand SK. Essential components of the Ti plasmid trb system, a type IV macromolecular transporter. J Bacteriol 1999; 181:5033 - 5041; PMID: 10438776
- Haase J, Lurz R, Grahn AM, Bamford DH, Lanka E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J Bacteriol 1995; 177:4779 - 4791; PMID: 7642506
- Lawley TD, Klimke WA, Gubbins MJ, Frost LS. F factor conjugation is a true type IV secretion system. FEMS Microbiol Lett 2003; 224:1 - 15; PMID: 12855161; http://dx.doi.org/10.1016/S0378-1097(03)00430-0
- Chen I, Christie PJ, Dubnau D. The ins and outs of DNA transfer in bacteria. Science 2005; 310:1456 - 1460; PMID: 16322448; http://dx.doi.org/10.1126/science.1114021
- Christie PJ. Type IV secretion: the Agrobacterium VirB/D4 and related conjugation systems. Biochim Biophys Acta 2004; 1694:219 - 234; PMID: 15546668; http://dx.doi.org/10.1016/j.bbamcr.2004.02.013
- Fronzes R, Schafer E, Wang L, Saibil HR, Orlova EV, Waksman G. Structure of a type IV secretion system core complex. Science 2009; 323:266 - 268; PMID: 19131631; http://dx.doi.org/10.1126/science.1166101
- Gomis-Rüth FX, Sola M, de la Cruz F, Coll M. Coupling factors in macromolecular type-IV secretion machineries. Curr Pharm Des 2004; 10:1551 - 1565; PMID: 15134575; http://dx.doi.org/10.2174/1381612043384817
- Llosa M, Bolland S, de la Cruz F. Genetic organization of the conjugal DNA processing region of the IncW plasmid R388. J Mol Biol 1994; 235:448 - 464; PMID: 8289274; http://dx.doi.org/10.1006/jmbi.1994.1005
- Moncalián G, Cabezon E, Alkorta I, Valle M, Moro F, Valpuesta JM, et al. Characterization of ATP and DNA binding activities of TrwB, the coupling protein essential in plasmid R388 conjugation. J Biol Chem 1999; 274:36117 - 36124; PMID: 10593894; http://dx.doi.org/10.1074/jbc.274.51.36117
- Okamoto S, Toyoda-Yamamoto A, Ito K, Takebe I, Machida Y. Localization and orientation of the VirD4 protein of Agrobacterium tumefaciens in the cell membrane. Mol Gen Genet 1991; 228:24 - 32; PMID: 1909421; http://dx.doi.org/10.1007/BF00282443
- Das A, Xie YH. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol 1998; 27:405 - 414; PMID: 9484895; http://dx.doi.org/10.1046/j.1365-2958.1998.00688.x
- Lee MH, Kosuk N, Bailey J, Traxler B, Manoil C. Analysis of F factor TraD membrane topology by use of gene fusions and trypsin-sensitive insertions. J Bacteriol 1999; 181:6108 - 6113; PMID: 10498725
- Rabel C, Grahn AM, Lurz R, Lanka E. The VirB4 family of proposed traffic nucleoside triphosphatases: common motifs in plasmid RP4 TrbE are essential for conjugation and phage adsorption. J Bacteriol 2003; 185:1045 - 1058; PMID: 12533481; http://dx.doi.org/10.1128/JB.185.3.1045-1058.2003
- Llosa M, Gomis-Ruth FX, Coll M, de la Cruz Fd F. Bacterial conjugation: a two-step mechanism for DNA transport. Mol Microbiol 2002; 45:1 - 8; PMID: 12100543; http://dx.doi.org/10.1046/j.1365-2958.2002.03014.x
- Tato I, Matilla I, Arechaga I, Zunzunegui S, de la Cruz F, Cabezon E. The ATPase activity of the DNA transporter TrwB is modulated by protein TrwA: implications for a common assembly mechanism of DNA translocating motors. J Biol Chem 2007; 282:25569 - 25576; PMID: 17599913; http://dx.doi.org/10.1074/jbc.M703464200
- Middleton R, Sjolander K, Krishnamurthy N, Foley J, Zambryski P. Predicted hexameric structure of the Agrobacterium VirB4 C terminus suggests VirB4 acts as a docking site during type IV secretion. Proc Natl Acad Sci USA 2005; 102:1685 - 1690; PMID: 15668378; http://dx.doi.org/10.1073/pnas.0409399102
- Draper O, Middleton R, Doucleff M, Zambryski PC. Topology of the VirB4 C terminus in the Agrobacterium tumefaciens VirB/D4 type IV secretion system. J Biol Chem 2006; 281:37628 - 37635; PMID: 17038312; http://dx.doi.org/10.1074/jbc.M606403200
- Gomis-Rüth FX, Moncalian G, Perez-Luque R, Gonzalez A, Cabezon E, de la Cruz F, et al. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature 2001; 409:637 - 641; PMID: 11214325; http://dx.doi.org/10.1038/35054586
- Hamilton CM, Lee H, Li PL, Cook DM, Piper KR, von Bodman SB, et al. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J Bacteriol 2000; 182:1541 - 1548; PMID: 10692358; http://dx.doi.org/10.1128/JB.182.6.1541-1548.2000
- Sastre JI, Cabezon E, de la Cruz F. The carboxyl terminus of protein TraD adds specificity and efficiency to F-plasmid conjugative transfer. J Bacteriol 1998; 180:6039 - 6042; PMID: 9811665
- Lujan SA, Guogas LM, Ragonese H, Matson SW, Redinbo MR. Disrupting antibiotic resistance propagation by inhibiting the conjugative DNA relaxase. Proc Natl Acad Sci USA 2007; 104:12282 - 12287; PMID: 17630285; http://dx.doi.org/10.1073/pnas.0702760104
- Garcillán-Barcia MP, Jurado P, Gonzalez-Perez B, Moncalian G, Fernandez LA, de la Cruz F. Conjugative transfer can be inhibited by blocking relaxase activity within recipient cells with intrabodies. Mol Microbiol 2007; 63:404 - 416; PMID: 17163977; http://dx.doi.org/10.1111/j.1365-2958.2006.05523.x
- Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 2008; 322:1843 - 1845; PMID: 19095942; http://dx.doi.org/10.1126/science.1165771
- Filutowicz M, Burgess R, Gamelli RL, Heinemann JA, Kurenbach B, Rakowski SA, et al. Bacterial conjugation-based antimicrobial agents. Plasmid 2008; 60:38 - 44; PMID: 18482767; http://dx.doi.org/10.1016/j.plasmid.2008.03.004
- Shoemaker NB, Guthrie EP, Salyers AA, Gardner JF. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol 1985; 162:626 - 632; PMID: 2985540
- Smith CJ, Macrina FL. Large transmissible clindamycin resistance plasmid in Bacteroides ovatus. J Bacteriol 1984; 158:739 - 741; PMID: 6725207
- Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev 2009; 73:775 - 808; PMID: 19946141; http://dx.doi.org/10.1128/MMBR.00023-09
- Hecht DW, Kos IM, Knopf SE, Vedantam G. Characterization of BctA, a mating apparatus protein required for transfer of the Bacteroides fragilis conjugal element BTF-37. Res Microbiol 2007; 158:600 - 607; PMID: 17720457; http://dx.doi.org/10.1016/j.resmic.2007.06.004