Abstract
Coordination of late mitotic events is crucial for the maintenance of genome stability and for the control of gene expression after cell division. Reversible protein phosphorylation regulates this process by de-phosphorylation of mitotic phospho-proteins in a sequential and coordinated manner: this allows an orderly sequence of events to take place during mitotic exit. We have identified Repo-Man/PP1 as a phosphatase complex that regulates temporally and spatially chromatin re-organization and nuclear envelope re-formation during anaphase-telophase.
Following CDK inactivation at anaphase onset, a pool of Repo-Man/PP1 localizes homogeneously to the anaphase chromatin, where it removes the major mitotic phosphorylations on Histone H3 (Thr3, Ser10 and Ser28). We have shown that this de-phosphorylation mediated by Repo-Man/PP1 is essential for the re-establishment of heterochromatin in post-mitotic cells.
A second pool of Repo-Man/PP1 targets to the periphery of chromosomes slightly later in anaphase. There, it contributes to the recruitment of Importin β to the chromatin. This fraction of Repo-Man appears to be important in the regulation of nuclear pores and lamina re-assembly since depletion of the complex leads to an abnormal lamina morphology and nuclear shape in G1 cells.
In summary Repo-Man/PP1 represents a molecular coupler between chromatin re-modeling and nuclear envelope (NE) re-formation that coordinates these processes during mitotic exit.Citation1
Introduction
Within the cell cycle, mitosis is a key process for the maintenance of genome stability. Progress through mitosis is highly regulated, and reversible protein phosphorylation is one of the key features that allows an orderly and timely execution of cell division.Citation2
Once sister chromatids have separated, the various biochemical and structural changes that have supported the entry into mitosis need to be reversed in order to start a new cell cycle.Citation3 This part of cell division is termed mitotic exit.
During mitotic exit, the compact structure of mitotic chromosomes needs to be dismantled and the chromatin de-condensed to allow transcription to be resumed in G1. At the same time, major rearrangements of the cytoskeleton take place to allow physical separation of the two daughter cells. Finally, in organisms in which the nuclear envelope (NE) breaks down during mitosis (open mitosis), the nuclear-cytoplasmic barrier must re-form. All these events take place in a brief time period (about 10 min in a human cell) and they are coordinated in space and time.Citation4
How the coordination of events during mitotic exit is achieved is an important open question, but the rapidly emerging picture is that this process is driven by a tug-of-war between kinases and phosphatases and their relative affinity for their substrates.Citation2,Citation5-Citation10 Thus, while prometaphase/metaphase is driven by high levels of CDK and low phosphatase activity, mitotic exit is dominated by a burst in phosphatase activity and the decreasing phosphorylation of CDK substrates.
In view of this, it is now of great interest to identify the phosphatases that regulate mitotic exit in space and time and to identify their specific substrates.Citation3,Citation11,Citation12
Chromatin Re-Organization During Mitotic Exit
During early mitosis, chromatin is organized as highly compacted structures known as mitotic chromosomes. At anaphase onset, when the sister chromatids start their journey to the opposite spindle poles, the chromatin undergoes several important changes that are essential for the reformation of a functional interphase nucleus. While several chromosomal proteins (histones and non-histones) are dephosphorylated but remain in place, others leave the chromosomes (e.g., the chromosomal passenger complex (CPC)) and still others are recruited to anaphase chromosomes.
Several studies have suggested that protein phosphatase 1 (PP1) is important for cells to progress from mitosis to G1. For example, PP1 mutants in Drosophila show abnormal sister chromatid segregation and excessive chromosome condensation.Citation13-Citation15 This phosphatase has also been suggested to promote histone dephosphorylation during mitotic exit.Citation16,Citation17 However, the PP1 targeting subunits responsible for these chromatin re-organisations are not known.
Repo-Man was identified as a nuclear protein that is a specific regulatory subunit for PP1γ,Citation18 which targets to the chromatin in anaphase. Repo-Man is mainly localized diffusely in the cytoplasm in early mitosis with a small fraction on mitotic chromosomes. At anaphase onset, Repo-Man is rapidly enriched on the segregating chromatin where it stays until the following entry into mitosis. Repo-Man localization and dynamics are controlled by phosphorylation. CDK1-CyclinB can phosphorylate Repo-Man in vitro. Mitotic Repo-Man (highly phosphorylated) has lower affinity for chromatin. Indeed, the behavior of the small chromosome-associated fraction in early mitosis is very dynamic (). Chemical inactivation of CDKs by roscovitine causes the re-localization of Repo-Man to chromosomes within a few minutes.Citation19,Citation20 Repo-Man mutants that cannot be fully phosphorylated by CDK localize to the chromatin even during prometaphase-metaphase. From anaphase onset and in interphase, Repo-Man is much less dynamic ().
Figure 1. Repo-Man/PP1 complex regulation and function in mitosis. (A and B) Analyses of the dynamic behavior of GFP:Repo-Man in prometaphase (A and A') and interphase (B and B') FRAP of HeLa cells transiently expressing GFP-Repo-Man in prometaphase (A and A') and interphase (B and B'). Cells were bleached using 488 nm laser line of a confocal microscope. Images were taken before bleaching and at the indicated time points after the end of the bleached pulse (dotted line) at every 2 sec. The bleached area is indicated by a blue circle. Graphs represent corresponding quantitative data for fluorescence recovery kinetics for the bleached area on chromosomes, (blue line) and unbleached area on chromosomes (red line) and, for the mitotic cells also the unbleached cytoplasms (green line). Fluorescence intensities of GFP-Repo-Man in the bleached region were measured and expressed as recovery rate. The values represent averages +/− SD from 5 (mitosis) and 5 (interphase) cells. (C) CDK and PP1 control the on/off-rate of Repo-Man onto the chromosomes.
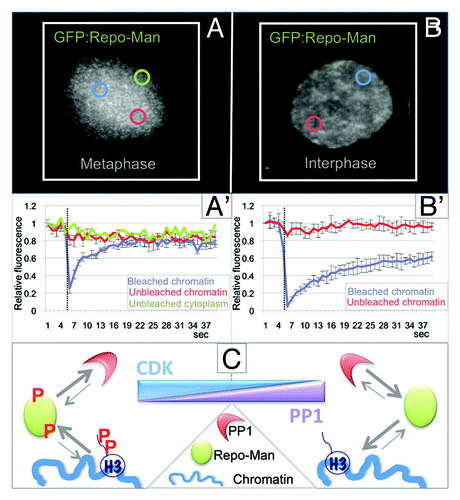
Besides regulating Repo-Man localization, CDK phosphorylation has a second important function: it regulates the binding of PP1 to Repo-Man. This phospho-regulation of the complex in early mitosis is extremely important, since it ensures that the Repo-Man/PP1 holoenzyme is not fully activated before anaphase onset. The binding of Repo-Man to PP1 and targeting of the complex to the chromosomes are both very brief and highly dynamic in early mitosis.Citation20 This transient base-line activity is probably necessary and sufficient to maintain the correct level of H3 phosphorylation on the chromosomes.Citation21 This is particularly relevant for the phosphorylation of Histone H3 Thr3, as it represents the docking site for the chromosome passenger complex (CPC) on mitotic chromosomes.Citation22-Citation25
Repo-Man is both a targeting subunit and substrate for PP1. This double regulation of the complex appears to be designed to act as a signal amplifier. In fact, small changes in CDK activity can result in enhanced binding of PP1 to the Repo-Man RVTF motif (Repo-Man docking site for PP1) and in Repo-Man de-phosphorylation. The resulting holoenzyme thus has an increased affinity for the chromatin and can effectively de-phosphorylate histone H3 and possibly other chromosomal substrates ().Citation20
The phosphorylation status of the Repo-Man regulatory subunit rather than PP1 itself directs the formation and activity of the Repo-Man/PP1 holoenzyme. This appears to contrast with results of a recent study, in which cell-cycle regulation of PP1 was found to occur at the level of the catalytic subunit, through PP1 phosphorylation and inhibitor-1 binding.Citation9 Our data suggest that there may be two different mechanisms for the regulation of PP1 activity in mitosis. It is possible that both are used according to the different substrates targeted.
At anaphase onset, Repo-Man/PP1 stably associates with the chromatin and promotes Histone H3 de-phosphorylation of Thr3,Citation26,Citation27 Ser10Citation28 and Ser28.Citation20,Citation21 The importance of these de-phosphorylations during mitotic exit is not completely understood. Removal of the Th3ph mark could be involved in the mechanism of CPC transfer to the spindle midzone, however clear demonstration of such a mechanism is still missing.
The removal of the Ser10 mark on histone H3 seems to be involved in the regulation of the binding of heterochromatin Protein 1 (HP1). HP1 recognizes methylation of H3K9 to direct heterochromatin formation.Citation29,Citation30 Aurora B phosphorylates Ser10 on H3 in prophase and this promotes the dissociation of HP1 from pericentric heterochromatin. Re-association of HP1 with chromatin can occur after dephosphorylation of H3S10p. We have shown that the Repo-Man PP1 complex is responsible for the phospho-switching regulation that mediates the re-establishment of heterochromatin in post-mitotic cells.
Nuclear Envelope Re-Formation
The coordination of chromatin de-condensation with nuclear reassembly during mitotic exit is not well understood.
Following CDK inactivation and the extraction of polyubiquitinated Aurora B by the AAA+ ATPase and ubiquitindependent chaperone p97,Citation31,Citation32 NPC re-assembly starts on the periphery of the segregating chromatin and then proceeds in a step-wise manner.Citation33 The first re-assembly events initiate as early as anaphase and much earlier than the recruitment of nuclear envelope vesicles. Detailed studies using the Xenopus in vitro system have made great contributions to clarify this process and to identify key players.Citation34 Moreover, recent studies on the temporal re-association dynamics of nucleoporins (Nups) during mitotic exit have added to our understanding of the post-mitotic assembly of the NPC. The first Nups to accumulate at the chromosome periphery are members of the Nup107-160 complex followed by a small fraction of Nup153 and Nup50.Citation35-Citation37 The recruitment of the Nup107-160 complex is mediated by Elys/MEL-28,Citation38-Citation41 which can associate to chromatin via its AT-hook.Citation42 ELYS/MEL-28 seems to represent an essential component in NPC re-assembly both in vitro and in vivo.Citation33
NPC reformation is regulated at several levels by importin β. In mitosis, Importin β binds to some of the Nups and prevents their association. During anaphase, RanGTP around chromatin directs the release of the Nups from this inhibitory complex with Importin β, therefore directing the spatial positioning of the NPC. As stated above, it is believed that the first NPC assembly step is conducted by the seeding of single copies of the Nup107-160 complex on the chromatin, mediated by the molecular adaptor ELYS/MEL-28. importin β negatively regulates the seeding at these sites and subsequent assembly steps are dependent on specific membrane components.Citation39,Citation43,Citation44 Depletion of ELYS/MEL-28 abolishes the assembly of pores at the chromatin periphery and causes the formation of annulate lamellae (membrane stacks of pores in the cytoplasm). Also fundamental to this process are the de-phosphorylation of nucleoporins, chromatin associated factors and NE membrane proteins.
A variety of evidence in the literature supports the involvement of PP1 and PP2A in nuclear membrane assembly at the M/G1 transition.Citation45-Citation47
PP1 appears to be the major mitotic lamin phosphatase responsible for removal of mitotic phosphates from lamin BCitation45 and the A-kinase anchoring protein AKAP149 recruits PP1 at the nuclear envelope (NE) upon somatic nuclear reformation in vitro.Citation46 PP1 targeting to the NE is also a prerequisite for assembly of B-type lamins. In Drosophila, reassembly of the NPC is blocked by the specific PP1/PP2A inhibitor okadaic acid.Citation47 Although the identity of the phosphatase responsible for de-phosphorylation of NUPs remains unknown, there are indications that at least NUP153 and NUP 50 can interact with PP1.Citation48
During our studies aimed at identifying the targets of Repo-Man/PP1 complex on anaphase chromatin we discovered a specific and direct interaction between Repo-Man and importin β. This binding is negatively regulated by Cdk phosphorylation of the N-terminal domain (aa 1–135) of Repo-Man. This interaction appears to be important for targeting at least a fraction of importin β to the periphery of the anaphase chromosomes and it seems to represent a direct structural function of Repo-Man rather than requiring catalytic activity of the Repo-Man/PP1 holoenzyme.
At this stage of mitotic exit, Repo-Man also interacts with NUP50 and NUP153.Citation49 However more work is required to understand how Repo-Man interacts with this subset of nucleoporins and to determine its biological relevance at the transition from mitosis to G1.
The importin β targeting function of Repo-man may represent an important step in NE re-assembly since it has been shown, at least in vitro, that Importin β levels are critical for proper NE re-assembly.Citation41,Citation44 In addition, it has been suggested that the pathway of nuclear pore complex assembly could be regulated at sequential points by transportin and importin β but also that other effectors could exist, particularly for the FG nucleoporins Nup358, Nup153 and Nup50.Citation39,Citation44 Our experiments showed that lack of Repo-Man compromises the process of NE reformation: Importin β is not properly recruited to the nuclear rim but form cytoplasmic aggregates that co-localize with NUPs (possibly annulate lamellae) and the nuclear lamina fails to form a smooth structure after cells have completed cytokinesis.
Current knowledge leads us to propose the following model. Very early during mitotic exit, the NUP107/160 complex is recruited to regions at the chromosome periphery via the chromatin binding protein ELYS/MEL-28. This is negatively regulated by importin β and transportin (). In the vicinity of these seeding regions, Repo-Man docks to the chromatin (directly or indirectly) and brings importin β and an early pool of NUP153/NUP50 (). In this respect Repo-Man could act either as an assembly factor for these FG NUPS or as a spacer by enriching some regions for importin β and preventing the seeding of more NUP107/160 complex.
Figure 2. Repo-Man/PP1 function in nuclear envelope re-assembly. Model for the involvement of Repo-Man in the regulation of NE re-assembly at the chromosome periphery of the anaphase chromatin. See text for details.
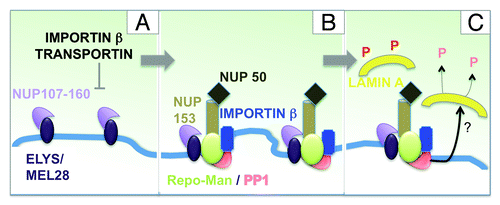
Although the binding and recruitment of importin β by Repo-Man seems to be independent of the catalytic activity of the Repo-Man/PP1 holoenzyme, it is plausible to assume that, once the complex is targeted to a localized region of the chromosome periphery, it could be involved in local de-phosphorylation processes either of NUPs or the lamina during the later steps of the nuclear re-assembly process ().
Further studies will be required to elucidate the global functions of Repo-Man at this stage of mitosis and to identify substrates critical for nuclear re-assembly. In particular it will be important to clarify the mechanisms and role of the interactions between Repo-Man and NUP153Citation50,Citation51 during mitotic exit. It will also be important to determine whether the fraction of Repo-Man bound to the nuclear periphery has a role in the organization and function of the interphase chromatin.
Future Prospective
Contrary to what is was believed, the emerging view is that protein phosphatase complexes show stringent and selective substrate specificity.Citation11,Citation52,Citation53 The modifications of chromatin during mitotic exit and the re-formation of a nuclear envelope both require the de-phosphorylation of several proteins in a local and timely manner. To date only three major PP1 targeting subunits have been found to be involved in these chromosome modifications in anaphase: Repo-Man,Citation18-Citation21 PNUTSCitation54,Citation55 and AKAP149.Citation46,Citation56,Citation57
Clearly other targeting subunits must be involved in this complex process and we can hypothesize that the anaphase chromosomes will give us more PP1 targeting subunits that will have specific functions regulating the transitional steps at the M/G1 boundary.
References
- Bickenson AF. Cell division: Repo-Man’s extra exit strategy. Nat Rev Mol Cell Biol 2011; 12:624; http://dx.doi.org/10.1038/nrm3197; PMID: 21915144
- Wurzenberger C, Gerlich DW. Phosphatases: providing safe passage through mitotic exit. Nat Rev Mol Cell Biol 2011; 12:469 - 82; http://dx.doi.org/10.1038/nrm3149; PMID: 21750572
- Queralt EUF, Uhlmann F. Cdk-counteracting phosphatases unlock mitotic exit. Curr Opin Cell Biol 2008; 20:661 - 8; http://dx.doi.org/10.1016/j.ceb.2008.09.003; PMID: 18845253
- Sullivan MMD, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol 2007; 8:894 - 903; http://dx.doi.org/10.1038/nrm2276; PMID: 17912263
- He E, Kapuy O, Oliveira RA, Uhlmann F, Tyson JJ, Novák B. System-level feedbacks make the anaphase switch irreversible. Proc Natl Acad Sci U S A 2011; 108:10016 - 21; http://dx.doi.org/10.1073/pnas.1102106108; PMID: 21617094
- Medema RH, Lindqvist A. Boosting and suppressing mitotic phosphorylation. Trends Biochem Sci 2011; 36:578 - 84; http://dx.doi.org/10.1016/j.tibs.2011.08.006; PMID: 21958687
- Uhlmann F, Bouchoux C, López-Avilés S. A quantitative model for cyclin-dependent kinase control of the cell cycle: revisited. Philos Trans R Soc Lond B Biol Sci 2011; 366:3572 - 83; http://dx.doi.org/10.1098/rstb.2011.0082; PMID: 22084384
- Chow JP, Poon RY, Ma HT. Inhibitory phosphorylation of cyclin-dependent kinase 1 as a compensatory mechanism for mitosis exit. Mol Cell Biol 2011; 31:1478 - 91; http://dx.doi.org/10.1128/MCB.00891-10; PMID: 21262764
- Wu JQ, Guo JY, Tang W, Yang CS, Freel CD, Chen C, et al. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat Cell Biol 2009; 11:644 - 51; http://dx.doi.org/10.1038/ncb1871; PMID: 19396163
- Bouchoux C, Uhlmann F. A quantitative model for ordered Cdk substrate dephosphorylation during mitotic exit. Cell 2011; 147:803 - 14; http://dx.doi.org/10.1016/j.cell.2011.09.047; PMID: 22078879
- Bollen M, Gerlich DW, Lesage B. Mitotic phosphatases: from entry guards to exit guides. Trends Cell Biol 2009; 19:531 - 41; http://dx.doi.org/10.1016/j.tcb.2009.06.005; PMID: 19734049
- Domingo-Sananes MR, Kapuy O, Hunt T, Novak B. Switches and latches: a biochemical tug-of-war between the kinases and phosphatases that control mitosis. Philos Trans R Soc Lond B Biol Sci 2011; 366:3584 - 94; http://dx.doi.org/10.1098/rstb.2011.0087; PMID: 22084385
- Dombrádi V, Axton JM, Barker HM, Cohen PT. Protein phosphatase 1 activity in Drosophila mutants with abnormalities in mitosis and chromosome condensation. FEBS Lett 1990; 275:39 - 43; http://dx.doi.org/10.1016/0014-5793(90)81434-P; PMID: 2175717
- Axton JM, Dombrádi V, Cohen PT, Glover DM. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell 1990; 63:33 - 46; http://dx.doi.org/10.1016/0092-8674(90)90286-N; PMID: 2170019
- Chen F, Archambault V, Kar A, Lio’ P, D’Avino PP, Sinka R, et al. Multiple protein phosphatases are required for mitosis in Drosophila. Curr Biol 2007; 17:293 - 303; http://dx.doi.org/10.1016/j.cub.2007.01.068; PMID: 17306545
- Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 2000; 102:279 - 91; http://dx.doi.org/10.1016/S0092-8674(00)00034-9; PMID: 10975519
- Murnion ME, Adams RR, Callister DM, Allis CD, Earnshaw WC, Swedlow JR. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem 2001; 276:26656 - 65; http://dx.doi.org/10.1074/jbc.M102288200; PMID: 11350965
- Trinkle-Mulcahy L, Andersen J, Lam YW, Moorhead G, Mann M, Lamond AI. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. J Cell Biol 2006; 172:679 - 92; http://dx.doi.org/10.1083/jcb.200508154; PMID: 16492807
- Vagnarelli P, Hudson DF, Ribeiro SA, Trinkle-Mulcahy L, Spence JM, Lai F, et al. Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nat Cell Biol 2006; 8:1133 - 42; http://dx.doi.org/10.1038/ncb1475; PMID: 16998479
- Vagnarelli P, Ribeiro S, Sennels L, Sanchez-Pulido L, de Lima Alves F, Verheyen T, et al. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev Cell 2011; 21:328 - 42; http://dx.doi.org/10.1016/j.devcel.2011.06.020; PMID: 21820363
- Qian J, Lesage B, Beullens M, Van Eynde A, Bollen M. PP1/Repo-man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal aurora B targeting. Curr Biol 2011; 21:766 - 73; http://dx.doi.org/10.1016/j.cub.2011.03.047; PMID: 21514157
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science 2010; 330:239 - 43; http://dx.doi.org/10.1126/science.1194498; PMID: 20929775
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 2010; 330:231 - 5; http://dx.doi.org/10.1126/science.1189435; PMID: 20705812
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 2010; 330:235 - 9; http://dx.doi.org/10.1126/science.1189505; PMID: 20705815
- Jeyaprakash AA, Basquin C, Jayachandran U, Conti E. Structural basis for the recognition of phosphorylated histone h3 by the survivin subunit of the chromosomal passenger complex. Structure 2011; 19:1625 - 34; http://dx.doi.org/10.1016/j.str.2011.09.002; PMID: 22032967
- Dai J, Higgins JM. Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle 2005; 4:665 - 8; http://dx.doi.org/10.4161/cc.4.5.1683; PMID: 15846065
- Dai J, Sultan S, Taylor SS, Higgins JM. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev 2005; 19:472 - 88; http://dx.doi.org/10.1101/gad.1267105; PMID: 15681610
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol 2001; 153:865 - 80; http://dx.doi.org/10.1083/jcb.153.4.865; PMID: 11352945
- Fischle W. In nucleo enzymatic assays for the identification and characterization of histone modifying activities. Methods 2005; 36:362 - 7; http://dx.doi.org/10.1016/j.ymeth.2005.03.008; PMID: 16085425
- Hirota T, Lipp JJ, Toh BH, Peters JM. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 2005; 438:1176 - 80; http://dx.doi.org/10.1038/nature04254; PMID: 16222244
- Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, et al. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature 2007; 450:1258 - 62; http://dx.doi.org/10.1038/nature06388; PMID: 18097415
- Meyer H, Drozdowska A, Dobrynin G. A role for Cdc48/p97 and Aurora B in controlling chromatin condensation during exit from mitosis. Biochem Cell Biol 2010; 88:23 - 8; http://dx.doi.org/10.1139/O09-119; PMID: 20130676
- Güttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol 2009; 10:178 - 91; http://dx.doi.org/10.1038/nrm2641; PMID: 19234477
- Antonin W, Ellenberg J, Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett 2008; 582:2004 - 16; http://dx.doi.org/10.1016/j.febslet.2008.02.067; PMID: 18328825
- Dultz E, Zanin E, Wurzenberger C, Braun M, Rabut G, Sironi L, et al. Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J Cell Biol 2008; 180:857 - 65; http://dx.doi.org/10.1083/jcb.200707026; PMID: 18316408
- Anderson DJ, Vargas JD, Hsiao JP, Hetzer MW. Recruitment of functionally distinct membrane proteins to chromatin mediates nuclear envelope formation in vivo. J Cell Biol 2009; 186:183 - 91; http://dx.doi.org/10.1083/jcb.200901106; PMID: 19620630
- Kutay U, Hetzer MW. Reorganization of the nuclear envelope during open mitosis. Curr Opin Cell Biol 2008; 20:669 - 77; http://dx.doi.org/10.1016/j.ceb.2008.09.010; PMID: 18938243
- Fernandez AG, Piano F. MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr Biol 2006; 16:1757 - 63; http://dx.doi.org/10.1016/j.cub.2006.07.071; PMID: 16950115
- Lau CK, Delmar VA, Chan RC, Phung Q, Bernis C, Fichtman B, et al. Transportin regulates major mitotic assembly events: from spindle to nuclear pore assembly. Mol Biol Cell 2009; 20:4043 - 58; http://dx.doi.org/10.1091/mbc.E09-02-0152; PMID: 19641022
- Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, et al. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep 2007; 8:165 - 72; http://dx.doi.org/10.1038/sj.embor.7400889; PMID: 17235358
- Walther TC, Alves A, Pickersgill H, Loïodice I, Hetzer M, Galy V, et al. The conserved Nup107-160 complex is critical for nuclear pore complex assembly. Cell 2003; 113:195 - 206; http://dx.doi.org/10.1016/S0092-8674(03)00235-6; PMID: 12705868
- Rasala BA, Ramos C, Harel A, Forbes DJ. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol Biol Cell 2008; 19:3982 - 96; http://dx.doi.org/10.1091/mbc.E08-01-0012; PMID: 18596237
- Rotem A, Gruber R, Shorer H, Shaulov L, Klein E, Harel A. Importin bea Regulates the Seeding of Chromatin with Initiation Sites for Nuclear Pore Assembly. Mol Biol Cell 2009; 20:4031 - 42; PMID: 19625448
- Harel A, Chan RC, Lachish-Zalait A, Zimmerman E, Elbaum M, Forbes DJ. Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol Biol Cell 2003; 14:4387 - 96; http://dx.doi.org/10.1091/mbc.E03-05-0275; PMID: 14551248
- Thompson LJ, Bollen M, Fields AP. Identification of protein phosphatase 1 as a mitotic lamin phosphatase. J Biol Chem 1997; 272:29693 - 7; http://dx.doi.org/10.1074/jbc.272.47.29693; PMID: 9368037
- Steen RL, Martins SB, Taskén K, Collas P. Recruitment of protein phosphatase 1 to the nuclear envelope by A-kinase anchoring protein AKAP149 is a prerequisite for nuclear lamina assembly. J Cell Biol 2000; 150:1251 - 62; http://dx.doi.org/10.1083/jcb.150.6.1251; PMID: 10995432
- Onischenko EA, Gubanova NV, Kiseleva EV, Hallberg E. Cdk1 and okadaic acid-sensitive phosphatases control assembly of nuclear pore complexes in Drosophila embryos. Mol Biol Cell 2005; 16:5152 - 62; http://dx.doi.org/10.1091/mbc.E05-07-0642; PMID: 16120647
- Moorhead GB, Trinkle-Mulcahy L, Nimick M, De Wever V, Campbell DG, Gourlay R, et al. Displacement affinity chromatography of protein phosphatase one (PP1) complexes. BMC Biochem 2008; 9:28; http://dx.doi.org/10.1186/1471-2091-9-28; PMID: 19000314
- Ullman KS, Shah S, Powers MA, Forbes DJ. The nucleoporin nup153 plays a critical role in multiple types of nuclear export. Mol Biol Cell 1999; 10:649 - 64; PMID: 10069809
- Mackay DR, Elgort SW, Ullman KS. The nucleoporin Nup153 has separable roles in both early mitotic progression and the resolution of mitosis. Mol Biol Cell 2009; 20:1652 - 60; http://dx.doi.org/10.1091/mbc.E08-08-0883; PMID: 19158386
- Mackay DR, Ullman KS. Coordinating postmitotic nuclear pore complex assembly with abscission timing. Nucleus 2011; 2; In press http://dx.doi.org/10.4161/nucl.2.4.16189; PMID: 21941107
- Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 2009; 33:537 - 45; http://dx.doi.org/10.1016/j.molcel.2009.02.015; PMID: 19285938
- Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 2010; 35:450 - 8; http://dx.doi.org/10.1016/j.tibs.2010.03.002; PMID: 20399103
- Lee JH, You J, Dobrota E, Skalnik DG. Identification and characterization of a novel human PP1 phosphatase complex. J Biol Chem 2010; 285:24466 - 76; http://dx.doi.org/10.1074/jbc.M110.109801; PMID: 20516061
- Landsverk HB, Kirkhus M, Bollen M, Küntziger T, Collas P. PNUTS enhances in vitro chromosome decondensation in a PP1-dependent manner. Biochem J 2005; 390:709 - 17; http://dx.doi.org/10.1042/BJ20050678; PMID: 15907195
- Steen RL, Collas P. Mistargeting of B-type lamins at the end of mitosis: implications on cell survival and regulation of lamins A/C expression. J Cell Biol 2001; 153:621 - 6; http://dx.doi.org/10.1083/jcb.153.3.621; PMID: 11331311
- Küntziger T, Rogne M, Folstad RL, Collas P. Association of PP1 with its regulatory subunit AKAP149 is regulated by serine phosphorylation flanking the RVXF motif of AKAP149. Biochemistry 2006; 45:5868 - 77; http://dx.doi.org/10.1021/bi060066s; PMID: 16669629