Abstract
Lamin A and the B-type lamins, lamin B1 and lamin B2, are translated as pre-proteins that are modified at a carboxyl terminal CAAX motif by farnesylation, proteolysis and carboxymethylation. Lamin A is further processed by proteolysis to remove the farnesyl, but B-type lamins remain permanently farnesylated. Two childhood diseases, Hutchinson Gilford Progeria Syndrome and restrictive dermopathy are caused by defects in the processing of lamin A, resulting in permanent farnesylation of the protein. Farnesyltransferase inhibitors, originally developed to target oncogenic Ras, have recently been used in clinical trials to treat children with Hutchinson Gilford Progeria Syndrome. Lamin B1 and lamin B2 play important roles in cell proliferation and organ development, but little is known about the role of farnesylation in their functions. Treating normal human fibroblasts with farnesyltransferase inhibitors causes the accumulation of unprocessed lamin B2 and lamin A and a decrease in mature lamin B1. Normally, lamins are concentrated at the nuclear envelope/lamina, but when farnesylation is inhibited, the peripheral localization of lamin B2 decreases as its nucleoplasmic levels increase. Unprocessed prelamin A distributes into both the nuclear envelope/lamina and nucleoplasm. Farnesyltransferase inhibitors also cause a rapid cell cycle arrest leading to cellular senescence. This study suggests that the long-term inhibition of protein farnesylation could have unforeseen consequences on nuclear functions.
Introduction
Hutchinson-Gilford Progeria Syndrome (HGPS) is a rare segmental premature aging disorder in which affected children acquire several phenotypic characteristics of accelerated aging. The majority of HGPS cases are due to de novo mutations in LMNA, the gene encoding the A-type lamins: lamin A (LA) and lamin C (LC).Citation1,Citation2 These two lamin isoforms are identical over the first 566 amino acids, but diverge in their carboxyl terminal tail domain sequences, due to alternative splicing between exon 10 and exon 11 creating a unique six amino acid tail sequence in LC and a different 98 amino acid tail sequence in LA. Whereas LC is translated as a mature protein, LA is translated as a preprotein, prelamin A (preLA), that is processed to produce the mature LA protein.Citation3 Processing of preLA is initiated by the addition of the C15 lipid farnesyl to the cysteine residue of a terminal CAAX motif by farnesyltransferase. Following addition of the farnesyl lipid, the three terminal residues of the CAAX sequence are proteolytically removed by the metallopeptidase Zmpste24/FACE1, and the new terminal cysteine carboxyl is methylated. The final processing step is the proteolytic removal of a 15 amino acid peptide including the farnesylated cysteine by Zmpste24 to produce mature LA.Citation4 The majority of HGPS cases are due to alternative splicing of the LMNA transcript initiated by a point mutation (G608G) in exon 11, which increases the recognition of a cryptic splice site. The alternatively spliced mRNA encodes a protein missing 50 amino acids, including the second proteolytic cleavage site for processing, resulting in a shorter form of LA that remains permanently farnesylated.Citation5-Citation8 The expression of this alternate form of LA, called progerin, causes changes in the shape of the nucleus, a loss of heterochromatin, altered mechanical properties of the nucleus, changes in gene expression and premature replicative senescence.Citation1,Citation2
Farnesylated progerin exerts a dominant toxicity on cells, which in humans and animal models is manifested as defects primarily in tissues of mesenchymal origin including bone, skin, fat and the cardiovascular system. The role of farnesylation in HGPS is supported by experiments demonstrating that most of the effects of progerin expression can be ameliorated in cultured cells and mouse models by preventing modification of the mutant protein.Citation8-Citation15 These findings have led to a clinical trial to treat HGPS patients with the farnesyltransferase inhibitor (FTI) lonafarnib.Citation16 The B-type lamins, lamin B1 (LB1) and lamin B2 (LB2), are also major farnesylated proteins, but unlike LA, there is no final proteolytic cleavage and the mature proteins remain farnesylated.Citation3 Two studies on the effects of FTIs on cell lines found either no defects in B-type lamin function or localization, or partial mislocalization of LB2 to cytoplasmic vesicles in a fraction of cells in one cell line.Citation17,Citation18 These findings were puzzling since inhibition of farnesylation should result in the accumulation of prelamins and mutations in the CAAX motif of LB1 or LB2 are known to mislocalize the proteins to the nucleoplasm.Citation19,Citation20 Little is known about the role of lamin farnesylation in lamin network formation at the nuclear envelope/lamina and the effect of FTIs on lamin network formation requires additional scrutiny.
The A- and B-type lamins form separate, but interacting, networks in the nucleus and depletion of LB1 by shRNA silencing can alter the remaining LB2 and LA/C networks.Citation21 Disease causing mutations in LA, including those that cause progeroid syndromes and muscular dystrophy, can also disrupt the organization of lamin networks.Citation22-Citation24 Together, these findings suggest that changes to one lamin network will also affect the other lamin networks. This means that some of the disease phenotypes ascribed to dysfunction in LA, may actually be mediated through changes in the B-type lamin networks. LB1 is also involved in the regulation of cell proliferation and both LB1 and LB2 are necessary for organ development, in particular the brain, in mice.Citation25-Citation28 In light of the recent clinical trial of FTIs on HGPS patients and the lack of information on the role of farnesylation in B-type lamin function, we re-examined the effects of FTIs on lamin processing and localization. Our data show that in normal human cells FTIs cause changes in the accumulation, processing and localization of both A- and B-type lamins. In addition, we confirm that FTIs induce rapid cell cycle arrest, which leads to senescence in proliferating cells.
Results
Several studies have examined the effects of FTIs on the farnesylation of preLA and progerin, the permanently farnesylated truncated form of LA.Citation29 However, only two studies have considered the effects of these drugs on the permanently farnesylated B-type lamins, and neither study found significant changes in their modification or localization.Citation17,Citation18 We recently demonstrated that silencing LB1 expression leads to the dramatic alteration of the remaining lamin meshworks in immortalized cells and rapid premature senescence in normal cells.Citation21,Citation28 In light of these findings, we re-examined the effects of altering the farnesylation state of B-type lamins in normal human fibroblasts. Normal human foreskin fibroblasts (BJ-5ta) were treated with the FTI L744,832 for 48 h and the A- and B-type lamins were examined by SDS-PAGE and immunoblotting. As previously shown, even a low concentration (0.5 μM) of FTI inhibited the farnesylation of LA leading to the appearance of the slower migrating preLA ().Citation9 We also observed a modest decrease in the amount of LB1 and the appearance of an additional band migrating more slowly than LB2, with a decrease in the amount of LB2 (). A previous study on the time course of processing of nascent B-type lamins found that LB2 first accumulated as a slower migrating precursor, but a precursor form of LB1 was never observed.Citation30 Therefore, it was likely that this new band was unmodified newly synthesized preLB2. For all of the following experiments, we chose to use the FTIs at 2.5 μM, since this concentration gave a robust response within 48 h and is within the ranges observed for mean plasma concentration of FTIs in clinical studies.Citation31,Citation32
Figure 1. FTIs induce the accumulation of prelamin B2. (A) The accumulation of preLB2 and preLA are dependent on the dose of FTI. SDS-PAGE and immunoblotting of lysates prepared from BJ-5ta cells treated for 48 h with the indicated concentrations of L-744,832. (B) The accumulation of preLB2 is time dependent. Cells treated with 2.5 μM L-744,832 for the indicated times. (C) The accumulation of preLB2 requires protein synthesis. Simultaneous addition of cycloheximide (10 μM; CHX) and FTI-L744,832 (2.5 μM) for 24 h. (D) Expression of wild type (WT) and a CAAX motif mutant (CVM) of LB2 in HeLa cells. Twenty-four h following transfection, cells were treated for an additional 48 h with 2.5 μM FTI-L744,832. (E) Total cell lysates from BJ-5ta cells treated FTI or vehicle were immunoblotted with a specific antibody to LB2 (LN43).
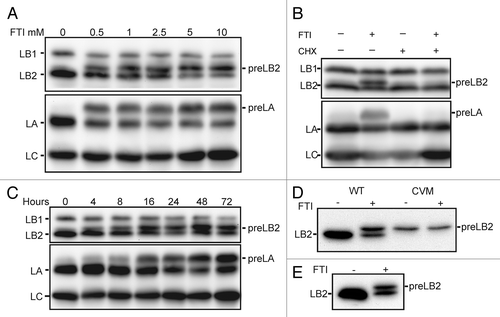
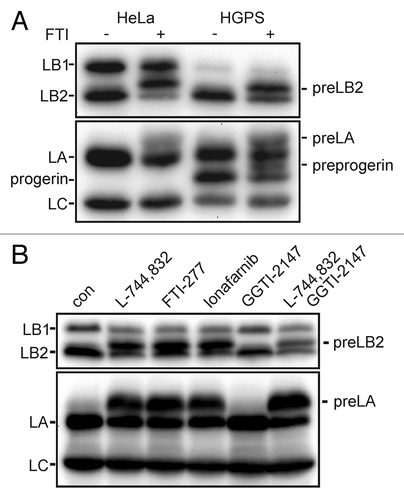
The accumulation of prelamins occurred within hours of FTI treatment. PreLB2 and preLA accumulation could be observed within four hours after addition of the FTI and approximately one half of the LB2 present was preLB2 by 16–24 h (). In a separate experiment, we measured lamin protein levels by immunoblotting after 4 d of FTI treatment and found that LB1 levels had decreased to 40–50% of untreated cell levels, while LB2 (preLB2 + LB2) was unchanged and LA (preLA + LA) had increased by approximately 75% (for example, compare 0 and 72 h in ). The addition of cycloheximide to the cells with the FTI completely abolished the accumulation of preLB2 and preLA supporting the idea that the slower migrating band was nascent preLB2 (). To address the possibility that the preLB2 band could be mature LB2 that had been post-translationally modified upon FTI addition, we mutated the carboxyl terminal CYVM farnesylation signal by deletion of the tyrosine. Wild type LB2 and the CVM mutant of LB2 were expressed as S-peptide tagged proteins in HeLa cells and detected by immunoblotting with an anti-S-peptide antibody (). The exogenously expressed wild type LB2 migrated similarly to the endogenous LB2 after FTI addition, whereas the CVM mutant LB2 migrated as a single band in the presence of the FTI. Together, the cycloheximide experiment and the expression of the mutant LB2 indicated that the FTI-induced band is preLB2. In order to confirm that the newly appearing band was preLB2 and not a proteolytic fragment of LB1, we immunoblotted protein lysates from control and FTI treated cells with a specific antibody for LB2 (). This specific antibody also recognized the FTI-induced band. The effect of FTIs on the accumulation of preLB2 was not restricted to BJ-5ta cells, but also occurred in HeLa cells and HGPS patient fibroblasts (). In addition, the effect was not an off-target effect of FTI L744,832, since preLB2 also accumulated in BJ-5ta cells treated with two other FTIs, FTI-277 and lonafarnib. The effects on all the lamins were specific to inhibition of farnesyltransferase as a geranylgeranyltransferase inhibitor GGTI-2147 did not cause the accumulation of prelamins (). When both an FTI and GGTI were added to the cells, preLB2 still accumulated, indicating that LB2 was not prenylated by geranylgeranyltransferase when farnesyltransferase was inhibited, as was previously reported for LA ().Citation33
Figure 2. Effect of FTI on lamins is not specific to cell type or drug. (A) PreLB2 accumulates in FTI treated HeLa cells and HGPS dermal fibroblasts (HGADFN136). (B) The accumulation of preLB2 occurs with different FTIs, but not with a GGTI. Each FTI was used at 2.5 μM and GGTI-2147 was used at 10 μM. All samples were treated for 48 h. In all panels A–D, equal amounts of protein were loaded in each gel lane.
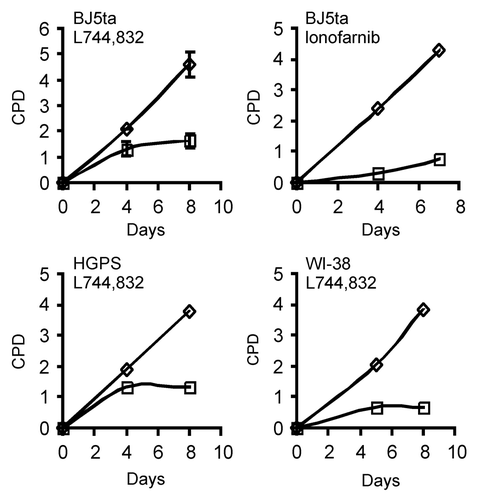
FTIs are known to induce apoptosis or cell cycle arrest in a variety of cells, although most studies have been performed in transformed cells.Citation34,Citation35 During the course of our experiments, we observed that FTI treated cells stopped proliferating within 48–96 h after addition of the drug. Also, the cells became larger and adopted a flattened morphology, and a small population of the cells became binucleate, suggesting that they had become senescent.Citation36 The decrease in LB1 levels that we observed by immunoblotting () were also consistent with the cells becoming senescent, as we and others have recently reported.Citation28,Citation37 In order to confirm that the FTIs were arresting proliferation, we measured the proliferation rate of FTI L744,832 treated cells over 8 d, finding that three different normal fibroblast cell lines stopped proliferating within 4 d after drug addition including BJ-5ta, HGPS patient dermal fibroblasts and WI-38 fetal lung fibroblasts (). Treatment of BJ-5ta cells treated with the FTI, lonafarnib, also led to strong suppression of proliferation (). In order to determine if the cells were becoming senescent, we counted cells expressing senescence associated β-galactosidase (SA-βgal) activity four days after drug addition in BJ-5ta and WI-38 treated with L744,832. A large fraction of cells in both cell lines were SA-βgal positive: BJ-5ta 45% vs. control 2.3% (n = 184, n = 222), WI-38 38% vs. control 2.1% (n = 221, n = 242). BJ-5ta cells treated with lonafarnib also increased expression of SA-βgal relative to control cells (38.5% vs. 1%; n = 192, n = 200). The mass of the cells also increased dramatically over the 8 d of treatment with FTI; treated BJ-5ta cells containing over 2-fold more protein than untreated cells (4.36 mg protein/107 cells vs. 2.38 mg protein/107 cells). Together, these results indicated that FTI treatment of normal fibroblasts rapidly induced senescence.
Figure 3. FTIs arrest the growth of normal fibroblasts in culture. The indicated cell lines were treated with 2.5 μM L744,832 or 2.5 μM lonafarnib and the proliferation rate of each culture was determined as described in Materials and Methods. Each point represents a pool of 4 plates of cells. Cell proliferation is expressed as cumulative population doublings (CPD). FTI treated cells (□). Untreated cells (◇). The error bars in the upper left graph are the standard deviation from 2 independent experiments.
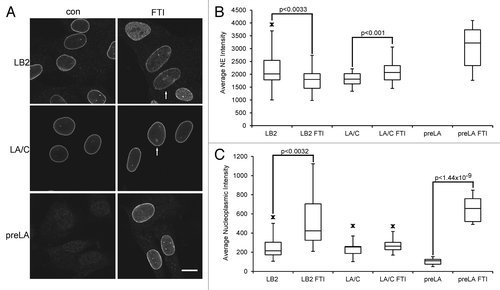
Several studies have suggested that the farnesylation of lamins is a crucial modification for their incorporation into the nuclear periphery.Citation38-Citation41 However, recent studies with a CAAX motif mutant or mice lacking farnesyltransferase expression in keratinocytes suggested that, at least for LA, incorporation into the lamina does not require farnesylation.Citation42,Citation43 The only two studies to examine the localization of enodogenous B-type lamins in FTI treated cells found either no altered localization of the proteins or the partial accumulation of LB2 in the cytoplasm in one cell line.Citation17,Citation18 We re-examined the localization of LA/C and LB2 in FTI treated cells by immunofluorescence in normal fibroblasts treated with FTI L744,832 for 40 h (). We were unable to identify a commercially available antibody to LB1 that did not also detect LB2 to some degree on immunoblots. Since these localization experiments required antibodies with high specificity, we excluded LB1 from our analyses. PreLA was undetectable in the nuclear envelope of untreated cells, but accumulated strongly in FTI treated cells (). In untreated cells, preLA could be detected weakly in the nucleoplasm and this localization increased significantly in FTI treated cells (). Total LA/C showed a small, but significant, increase in intensity at the nuclear periphery after FTI treatment (), consistent with the increase in LA observed by immunoblotting (). However, no significant change was seen in LA/C localization in the nucleoplasm (). In contrast, FTI treatment caused a significant decrease in the intensity of LB2 at the nuclear periphery with a corresponding increase in nucleoplasmic accumulation (). It is likely that the nucleoplasmic signal that accumulated was due to the newly synthesized preLB2 and not movement of mature LB2 from the periphery into the nucleoplasm. We also observed the formation of donut shaped nuclei similar to those reported previously in FTI treated cells ().Citation8,Citation20
Figure 4. Localization of lamins by indirect immunofluorescence in FTI-treated cells. BJ-5ta cells were treated with 2.5 μM L744,832 for 40 h prior to fixation as described in Materials and Methods. (A) Representative images of cells immunostained to localize LA/C, preLA and LB2. Bar = 10 μm. Arrows indicate donut shaped nuclei. (B) Quantification of the nuclear envelope/lamina (NE) signal intensity for each lamin. (C) Quantification of the nucleoplasmic intensity for each lamin. For the measurements in (B) and (C), the number of nuclei scanned for each lamin was: LA/C (13), LA/C FTI (17), preLA (15), preLA FTI (13), LB1 (14), LB1 FTI (13), LB2 (20) and LB2 FTI (14). Arrow indicates the minimum or maximum outliers.
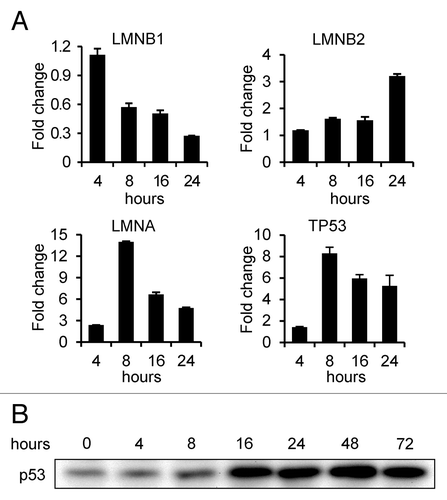
Because of the rapid onset of senescence in FTI treated cells and the changes in lamin expression, we performed qRT-PCR analyses of RNA isolated from cells 4, 8, 16 and 24 h following FTI addition to determine how lamin gene transcription was affected by the FTI treatment (). Each lamin gene had a unique pattern of expression. LMNB1 mRNA levels began to change between 4 h and 8 h after FTI addition, decreasing to 27% of control cell levels by 24 h. The decrease in LMNB1 mRNA levels within 8 h is consistent with the decrease in LB1 protein levels and provides an explanation for our inability to detect preLB1 by immunoblotting (see ). In contrast, LMNA mRNA levels increased over 2-fold within 4 h and 14-fold by 8 h, dropping to 4.7-fold by 24 h. LMNB2 mRNA levels remained similar to control levels for the first 16 h and increased modestly to 3-fold above controls between 16 h and 24 h. The regulation of cell proliferation by FTIs involves a p53-dependent mechanism.Citation34 The induction of TP53 transcription between 4 and 8 h () and the increase in p53 protein levels between 8 and 16 h () following FTI treatment is consistent with a p53 mediated induction of cell cycle arrest and senescence seen in the experiments presented here. The dramatic increase in LMNA mRNA levels between 4 h and 8 h after FTI treatment is also consistent with the regulation of LMNA by p53.Citation44
Discussion
Lamins are major structural proteins of the nucleus with known roles in chromatin organization, nuclear envelope structure and interactions between the nucleus and cytoplasm. Multiple interactions between lamins and other nuclear proteins have been identified, suggesting lamins play important roles in transcription, replication and DNA repair.Citation45 Several post-translational modifications of lamins, including phosphorylation, SUMOylation, ADP ribosylation and farnesylation are known and have been shown to be involved in regulating lamin localization, lamina assembly and lamin interactions with associated proteins.Citation4,Citation46-Citation48 In this study we examine the effects of inhibiting lamin farnesylation on B-type lamin expression and modification using FTIs in normal human fibroblasts. Our results identify the accumulation and mislocalization of preLB2 and the downregulation of LB1 as consequences of inhibiting farnesyltransferase. Two previous studies have examined the effects of FTIs on B-type lamins. The first found that the inhibitor had no effect on B-type lamin localization in CHO cells even when farnesylation was completely inhibited.Citation17 A second study found that LB2 accumulated normally in most tumor cell lines after FTI treatment, but one tumor cell line accumulated cytoplasmic vesicles containing LB2.Citation18 Notably, neither previous study identified the accumulation of preLB2 as a consequence of farnesyltransferase inhibition. We also demonstrate that FTIs cause a rapid proliferation arrest and induction of senescence in normal fibroblasts leading to a decrease in LB1 expression.
Two progeroid disorders have been identified where the permanent farnesylation of LA is a causative factor in disease progression: HGPS and restrictive dermopathy (RD).Citation7,Citation49 Unlike the mutation in LMNA that causes HGPS, RD is caused by a deficiency in ZMPSTE24, the enzyme which catalyzes the proteolytic removal of the farnesylated carboxyl terminal peptide of preLA during processing. FTIs ameliorate many of the disease phenotypes in mouse models of HGPS and RD.Citation8-Citation15 While the FTI results strongly support the idea that the accumulation of farnesylated progerin is important for developing HGPS, mice expressing a non-farnesylatable form of progerin with a cysteine to serine substitution in the CAAX motif (CSIM to SSIM) also develop a severe progeria.Citation50 However, mice expressing a non-farnesylatable form of progerin in which the CAAX motif was altered by removal of the penultimate isoleucine residue (CSIM to CSM) were overtly normal.Citation51 These findings suggest that, in addition to permanent farnesylation, the progerin structure may also be important in the disease etiology. In support of the idea that accumulation of a form of LA without the normally processed carboxyl terminal sequence can cause disease, knock-in mice expressing exclusively non-farnesylated preLA, in the absence of LC, develop cardiomyopathy with no evidence of progeroid features.Citation43 In the first trial of FTIs involving children with HGPS, the FTI lonafarnib appears to improve some aspects of the disease in some of the children.Citation16 Since HGPS is a progressive disease, affected children will likely require long-term treatment with FTIs to prevent the accumulation of toxic levels of farnesylated preLA. One shortcoming of the recent clinical trial as noted by the authors is the lack of a reliable pharmacodynamic marker that can be correlated with the clinical responses. The biomarker used in the HGPS study and in many cancer studies employing FTIs is the small farnesylated heat shock protein HDJ-2.Citation52 Our studies suggest that the farnesylation of LB2 may be a more suitable biomarker for HGPS trials, since it is closely related to progerin in structure, function and cellular localization. In addition, the kinetics of preLA and preLB2 protein accumulation after FTI treatment appears to be similar.
Although FTIs have been in use for the treatment of cancer for at least a decade, the long-term effects of inhibiting the processing or activity of several important classes of prenylated proteins are unknown. FTIs were designed to inhibit modification of Ras, but are also known to inhibit the modification of several Ras GTPases, RhoB, centromere associated proteins, lamins and other proteins.Citation53 Although FTIs are well tolerated in animal models and in human patients, FTI treatment of mice or cells in culture leads to the formation of donut-shaped nuclei due to a centrosome separation defect.Citation8,Citation20 FTIs induce the degradation of the centrosome scaffold protein pericentrin, which itself is not farnesylated, leading to defects in bipolar mitotic spindle formation.Citation20 Interestingly, the farnesylated protein LB1 plays a role in forming the donut shaped nuclei. Other studies have shown that LB1 is required for normal mitotic spindle formation and farnesylation of the protein may be crucial for this function.Citation54 It is also likely that farnesylation is not required in all cells equally. Mice with a keratinocyte specific conditional knockout of the β-subunit of farnesyltransferase (Fntb) have defects in hair follicles, but not in the interfollicular epidermis. Although the epidermis exhibited increased apoptosis, keratinocytes were apparently replaced efficiently enough to support normal skin development. However, keratinocytes isolated from these mice do not proliferate in culture, suggesting that in vitro culture could possibly render some cells more sensitive to the inability to farnesylate proteins.Citation42 Hepatocyte specific farnesyltransferase knockout mice develop hepatocellular disease and primary hepatocytes have a high frequency of misshapen nuclei with nuclear blebs.Citation55 Silencing LB1 expression in many cell types also leads to the formation of misshapen nuclei and nuclear blebs with structural alterations of the remaining lamin networks.Citation21 It is possible that the nuclear defects in hepatocytes from the farnesyltransferase knockout mouse are due to the mislocalization or decreased expression of LB1 in the absence of farnesylation.
In contrast to LMNA for which over 400 mutations have been identified causing at least 12 diseases including muscular dystrophies, lipodystrophies, peripheral neuropathy and progeroid disorders, few human diseases have been linked to mutations in the B-type lamins.Citation2 Duplications in LMNB1 cause an adult onset autosomal-dominant leukodystrophy and mutations in LMNB2 are implicated in an acquired partial lipodystrophy, Barraquer-Simons disease.Citation56 B-type lamins have also been shown to be critical components for organogenesis in mice, particularly of the brain, although they appear to be dispensable for the development of skin and hair.Citation25-Citation27,Citation57 In adult Xenopus neurons, LB2 plays a role in axon maintenance by promoting mitochondrial function.Citation58 In this study, we show that preLB2 accumulates in the nuclei of cells treated with FTIs, but we cannot determine if the accumulation of preLB2 has any effects on cell function because of the added complication of FTI-induced senescence. However, if correct localization and assembly into the lamina is required for LB2 function, the nucleoplasmic accumulation of preLB2 may have a deleterious effect. It would be interesting to investigate the effect of a non-farnesylatable LB2 on development in a mouse model as has been done for LA.Citation43
Another consequence of FTI treatment of cells is a rapid cell cycle arrest, but a single mechanism to explain the effects of FTIs on proliferation and apoptosis both normal and tumor cells has not been identified.Citation53 In our experiments, FTIs induce a rapid cell cycle arrest that appears to be mediated by p53.Citation59 The activation of p53 appears to occur between 4 and 8 h following FTI addition, at the same time that preLB2 is accumulating. However, we cannot at this time conclude that the accumulating preLB2 has any role in p53 activation. We recently showed that LB1 is important for maintaining cell proliferation and that the steady-state level of this lamin decreases as cells become senescent.Citation28 Silencing of LB1 expression by shRNA rapidly induces senescence, so the decrease in LB1 levels during senescence may reinforce the senescent state. Cells treated with FTIs also show a lower steady-state concentration of LB1 protein and LMNB1 mRNA levels decrease within hours after FTI addition. This observation supports our finding that FTI treated cells rapidly become senescent.
The carboxyl terminal modification of LA, LB1 and LB2 by farnesylation and its role in membrane targeting has been known for many years, although the role of this added lipid in lamin function remains unknown.Citation4 A trivial explanation for the farnesylation is that the lipid is required for close association of lamins with the inner membrane of the nuclear envelope and assembly into the polymeric lamin structure present within the nuclear lamina. Why then is the farnesyl removed from LA, but retained by LB1 and LB2? The localization of exogenously expressed lamins with mutations in the CAAX motif or the treatment of cells with FTIs suggests that LA and the B-type lamins may have different pathways for assembly into the lamina. Farnesylation of LA does not appear to be absolutely required for LA assembly into the lamina, but modification is required for B-type lamin localization.Citation42,Citation43 A- and B-type lamins form separate, but interacting networks, and these different assembly paths may be required to keep the networks separated.Citation21 In further support of the idea that A- and B-type lamins form separate networks, blocking farnesylation of transfected progerin by CAAX motif mutation or FTI treatment leads to the redistribution of LA and LC away from the nuclear envelope, but does not disrupt the localization of LB1.Citation60 Further studies on the farnesylation of the lamins will be needed to determine the functional role or roles of this modification in lamin-dependent processes.
Materials and Methods
Cell culture
BJ-5ta human foreskin fibroblasts immortalized with hTERT (ATCC CRL-4001) were cultured in a 4:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM; Gibco) and Medium 199 (Gibco) supplemented with 10% fetal bovine serum (FBS; Hyclone), 100 units/ml penicillin and 100 μg/ml streptomycin (Pen-Strep) and 10 μg/ml hygromycin (complete medium). WI-38 human fetal lung fibroblasts (ATCC CCL-75) were cultured in Eagle’s Minimum Essential Medium (MEM; Gibco), 10% FBS and Pen-Strep. HGPS patient fibroblasts (HGADFN136; Progeria Research Foundation) were cultured in DMEM, 10% FBS and Pen-Strep. HeLa cells (ATCC CCL-2) were cultured in DMEM with 10% FBS and Pen-Strep. All cells were maintained in a humidified incubator at 37°C with 5% CO2. For expression of S-peptide tagged LB2 and LB2(CVM), DNA encoding amino acids 2–600 of human LB2 was inserted into pCDNA3.1 that was engineered to express an S-peptide at the N-terminus of the inserted cDNA sequence. The purified DNA was introduced into HeLa cells by electroporation.
Chemicals
Prenyltransferase inhibitors used in this study are the FTIs: L-744,832 (ENZO Life Sciences;BML-G242), FTI-277 (Calbiochem;344555), lonafarnib (a gift from Schering-Plough Research Institute) and the geranylgeranyltransferase inhibitor GGTI-2147 (Calbiochem;345885). All of the inhibitors were dissolved in dimethylsulfoxide (DMSO). Cycloheximide (Sigma; C7698) was dissolved in ethanol. For controls in all experiments, an equal volume of vehicle was added.
Antibodies
The antibody to detect LA and LC by immunoblotting and immunofluorescence was a mouse monoclonal prepared in our laboratory (5G4). It was used at a 1:4000 dilution for immunoblotting and 1:2000 for immunofluorescence. A mouse monoclonal antibody prepared in our laboratory to LB1 and LB2 (2B2) was used for immunoblotting at 1:2000. LB2 was detected by immunofluorescence and immunoblotting using a mouse monoclonal antibody (Abcam; LN43) at a 1:400 dilution. Rabbit anti-preLA was a gift from Dr. Michael Sinensky and was used at 1:2000.Citation61 Mouse monoclonal antibodies to p53 (Santa Cruz Biotechnology; DO-1) was used for immunoblotting at a 1:200 dilution. The anti-S-tag antibody (Novagen; 71549) was used for immunoblotting at a 1:5000 dilution.
Gel electrophoresis
Cells were collected by trypsinization followed by quenching in complete culture medium containing 10% FBS and washed twice with ice cold phosphate buffered saline (PBS) by centrifugation at 200 × g for 5 min and resuspension. The final pellet was resuspended in SDS-polyacrylamide gel (SDS-PAGE) sample buffer and the protein concentration determined using the BCA method (Pierce;23225). SDS-PAGE gels were prepared as described.Citation62 At the completion of electrophoresis, the proteins were transferred to nitrocellulose (Millipore) using a Genie Blotter (Idea Scientific, Minneapolis MN). After staining with 0.2% Ponceau S in 3% acetic acid and destaining, the nitrocellulose was blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBST).
Immunoblotting
Antibodies for immunoblotting were diluted in 5% non-fat dry milk in TBST and incubated with the nitrocellulose overnight at 4°C. The blots were washed 5 × with TBST before incubation with horseradish peroxidase conjugated goat anti-mouse or goat anti-rabbit diluted 1:50,000 from a 2 mg/ml stock in 5% non-fat dry milk in TBST for 1 h at room temperature. The blots were finally washed 5 × with TBST and incubated for 3 min in SuperSignal West Pico Chemiluminescent Substrate (Pierce;34078). Images were collected on a Kodak Image Station 440CF and quantification of gel images was performed with Kodak Image software (Kodak).
Indirect immunofluorescence
Cells grown on glass coverslips were fixed for immunofluorescence by quickly washing once in room temperature PBS, followed by incubation in freshly prepared 2% formaldehyde in PBS for 20 min. The fixed cells were washed twice in PBS, blotted to remove excess buffer and immersed in 100% methanol at -20°C for 4 min. After washing in PBS, the fixed and permeabilized cells were blocked for 6 h in 2% bovine serum albumin, 1% normal goat serum in PBS. Antibodies were diluted in the blocking buffer and incubated overnight on the cells at 4°C. The primary antibodies were removed by 3 × 5 min washes with PBS. Secondary antibodies were Alexa 488 goat anti-mouse F(ab’)2 or Alexa 488 goat anti-rabbit (Molecular Probes;A-11017,A-11008) diluted 1:800 from a 2 mg/ml stock in blocking buffer and incubated on the cells for 1hr at room temperature. The stained cells were observed with a Zeiss LSM 510META (Carl Zeiss) using a Plan-Apochromat 63 × /1.4 oil objective. Images for each lamin were collected with identical microscope settings (laser intensity, exposure time, gain) for each pair of samples (+/− FTI). The intensity of the nuclear envelope and nucleoplasmic signals were determined by using the profile function of the LSM software with a 5 pixel-wide line scan. The raw pixel intensity data from a 0.2 μm segment corresponding to the peak of the nuclear envelope signal at two points for each nucleus were averaged to obtain nuclear envelope peak intensities. The average nucleoplasmic intensity was obtained by averaging the raw pixel intensity values over a 4μm segment of the line scan in the center of each nucleus. Background intensities for each scan were obtained from 1 μm segments at the beginning and end of each line scan and were subtracted from the nuclear envelope and nucleoplasm values. The statistical significance of the data was determined using a two-tailed Student’s t-test.
Proliferation assays
Cells were plated on 10 cm culture plates at a density of 4 × 105 cells/plate, with 4 plates used for each condition or time point. The cultures were incubated for 3–5 d and the cells collected by trypsinization. The cells pooled from 4 plates were counted in a hemocytometer and a portion of the cells was replated on 4 plates at 4 × 105 cells/plate. The same procedure was repeated for successive passages. The number of population doublings at each passage was calculated using the formula: population doublings = log(number of cells harvested – number of cells plated)/log2. In some experiments, the cells that were not replated were denatured in 1X SDS-PAGE sample buffer for electrophoresis. Senescence associated β-galactosidase activity was measured as described.Citation63
Quantitative analysis of mRNA
Total RNA from two 10 cm plates of control or FTI-treated cells at each time point was harvested using TRIzol (Life Technologies;10296) and subjected to DNase I (Promega;M610A) treatment (3 u/μl of reaction mixture) followed by an additional precipitation with TRIzol. An ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington DE) was used to assess the quality and concentration of the RNA preparations. RT-PCR reactions were performed with the SuperScript VILO cDNA Synthesis kit 20 (Invitrogen). For quantitative reverse transcription polymerase chain reactions (qRT-PCR) we used the Qiagen QuantiFast Multiplex RT-PCR Kit. Each experiment was performed in duplicate and qPCR reactions for each gene were performed in triplicate. Real-time PCR p-values were determined by Student's T-test comparing ΔCT for the target genes to the average ΔCT for the reference genes.Citation64
| Abbreviations: | ||
| FTI | = | farnesyltransferase inhibitor |
| LA | = | lamin A |
| LC | = | lamin C |
| LB1 | = | lamin B1 |
| LB2 | = | lamin B2 |
| HGPS | = | Hutchinson-Gilford Progeria Syndrome |
| RD | = | restrictive dermopathy |
| PD | = | population doubling |
| CPD | = | cumulative population doubling |
| qRT-PCR | = | quantitative reverse transcription-polymerase chain reaction |
Acknowledgments
This work was supported by grants from the Progeria Research Foundation and by NCI grant R01CA031760 to R.D.G.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gonzalez JM, Pla D, Perez-Sala D, Andres V. A-type lamins and Hutchinson-Gilford progeria syndrome: pathogenesis and therapy. [Schol Ed] Front Biosci (Schol Ed) 2011; 3:1133 - 46; http://dx.doi.org/10.2741/216; PMID: 21622261
- Reddy S, Comai L. Lamin A, farnesylation and aging. Exp Cell Res 2012; 318:1 - 7; http://dx.doi.org/10.1016/j.yexcr.2011.08.009; PMID: 21871450
- Adam SA, Goldman RD. Insights into the differences between the Aand B-type nuclear lamins. Adv Biol Regul 2012; 52:108 - 13; http://dx.doi.org/10.1016/j.advenzreg.2011.11.001; PMID: 22119859
- Rusiñol AE, Sinensky MS. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J Cell Sci 2006; 119:3265 - 72; http://dx.doi.org/10.1242/jcs.03156; PMID: 16899817
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003; 300:2055; http://dx.doi.org/10.1126/science.1084125; PMID: 12702809
- Dechat T, Shimi T, Adam SA, Rusinol AE, Andres DA, Spielmann HP, et al. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc Natl Acad Sci U S A 2007; 104:4955 - 60; http://dx.doi.org/10.1073/pnas.0700854104; PMID: 17360326
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003; 423:293 - 8; http://dx.doi.org/10.1038/nature01629; PMID: 12714972
- Glynn MW, Glover TW. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum Mol Genet 2005; 14:2959 - 69; http://dx.doi.org/10.1093/hmg/ddi326; PMID: 16126733
- Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, Moulson CL, et al. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc Natl Acad Sci U S A 2005; 102:12873 - 8; http://dx.doi.org/10.1073/pnas.0505767102; PMID: 16129834
- Mallampalli MP, Huyer G, Bendale P, Gelb MH, Michaelis S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A 2005; 102:14416 - 21; http://dx.doi.org/10.1073/pnas.0503712102; PMID: 16186497
- Yang SH, Bergo MO, Toth JI, Qiao X, Hu Y, Sandoval S, et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci U S A 2005; 102:10291 - 6; http://dx.doi.org/10.1073/pnas.0504641102; PMID: 16014412
- Yang SH, Meta M, Qiao X, Frost D, Bauch J, Coffinier C, et al. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest 2006; 116:2115 - 21; http://dx.doi.org/10.1172/JCI28968; PMID: 16862216
- Capell BC, Erdos MR, Madigan JP, Fiordalisi JJ, Varga R, Conneely KN, et al. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A 2005; 102:12879 - 84; http://dx.doi.org/10.1073/pnas.0506001102; PMID: 16129833
- Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, Tavarez UL, et al. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci U S A 2008; 105:15902 - 7; http://dx.doi.org/10.1073/pnas.0807840105; PMID: 18838683
- Fong LG, Frost D, Meta M, Qiao X, Yang SH, Coffinier C, et al. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 2006; 311:1621 - 3; http://dx.doi.org/10.1126/science.1124875; PMID: 16484451
- Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A 2012; 109:16666 - 71; http://dx.doi.org/10.1073/pnas.1202529109; PMID: 23012407
- Dalton MB, Fantle KS, Bechtold HA, DeMaio L, Evans RM, Krystosek A, et al. The farnesyl protein transferase inhibitor BZA-5B blocks farnesylation of nuclear lamins and p21ras but does not affect their function or localization. Cancer Res 1995; 55:3295 - 304; PMID: 7614464
- Mégnin-Chanet F, Lavelle F, Favaudon V. The farnesyl transferase inhibitor RPR-130401 does not alter radiation susceptibility in human tumor cells with a K-Ras mutation in spite of large changes in ploidy and lamin B distribution. BMC Pharmacol 2002; 2:2; http://dx.doi.org/10.1186/1471-2210-2-2; PMID: 11929613
- Kitten GT, Nigg EA. The CaaX motif is required for isoprenylation, carboxyl methylation, and nuclear membrane association of lamin B2. J Cell Biol 1991; 113:13 - 23; http://dx.doi.org/10.1083/jcb.113.1.13; PMID: 2007618
- Verstraeten VL, Peckham LA, Olive M, Capell BC, Collins FS, Nabel EG, et al. Protein farnesylation inhibitors cause donut-shaped cell nuclei attributable to a centrosome separation defect. Proc Natl Acad Sci U S A 2011; 108:4997 - 5002; http://dx.doi.org/10.1073/pnas.1019532108; PMID: 21383178
- Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 2008; 22:3409 - 21; http://dx.doi.org/10.1101/gad.1735208; PMID: 19141474
- Burke B, Mounkes LC, Stewart CL. The nuclear envelope in muscular dystrophy and cardiovascular diseases. Traffic 2001; 2:675 - 83; http://dx.doi.org/10.1034/j.1600-0854.2001.21001.x; PMID: 11576443
- Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A 2004; 101:8963 - 8; http://dx.doi.org/10.1073/pnas.0402943101; PMID: 15184648
- Taimen P, Pfleghaar K, Shimi T, Möller D, Ben-Harush K, Erdos MR, et al. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci U S A 2009; 106:20788 - 93; http://dx.doi.org/10.1073/pnas.0911895106; PMID: 19926845
- Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, et al. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci U S A 2010; 107:5076 - 81; http://dx.doi.org/10.1073/pnas.0908790107; PMID: 20145110
- Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH 2nd, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell 2011; 22:4683 - 93; http://dx.doi.org/10.1091/mbc.E11-06-0504; PMID: 21976703
- Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, et al. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 2011; 334:1706 - 10; http://dx.doi.org/10.1126/science.1211222; PMID: 22116031
- Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 2011; 25:2579 - 93; http://dx.doi.org/10.1101/gad.179515.111; PMID: 22155925
- Young SG, Meta M, Yang SH, Fong LG. Prelamin A farnesylation and progeroid syndromes. J Biol Chem 2006; 281:39741 - 5; http://dx.doi.org/10.1074/jbc.R600033200; PMID: 17090536
- Lehner CF, Fürstenberger G, Eppenberger HM, Nigg EA. Biogenesis of the nuclear lamina: in vivo synthesis and processing of nuclear protein precursors. Proc Natl Acad Sci U S A 1986; 83:2096 - 9; http://dx.doi.org/10.1073/pnas.83.7.2096; PMID: 3515346
- Kieran MW, Packer RJ, Onar A, Blaney SM, Phillips P, Pollack IF, et al. Phase I and pharmacokinetic study of the oral farnesyltransferase inhibitor lonafarnib administered twice daily to pediatric patients with advanced central nervous system tumors using a modified continuous reassessment method: a Pediatric Brain Tumor Consortium Study. J Clin Oncol 2007; 25:3137 - 43; http://dx.doi.org/10.1200/JCO.2006.09.4243; PMID: 17634493
- Castaneda C, Meadows KL, Truax R, Morse MA, Kaufmann SH, Petros WP, et al. Phase I and pharmacokinetic study of lonafarnib, SCH 66336, using a 2-week on, 2-week off schedule in patients with advanced solid tumors. Cancer Chemother Pharmacol 2011; 67:455 - 63; http://dx.doi.org/10.1007/s00280-010-1488-5; PMID: 20972873
- Varela I, Pereira S, Ugalde AP, Navarro CL, Suárez MF, Cau P, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med 2008; 14:767 - 72; http://dx.doi.org/10.1038/nm1786; PMID: 18587406
- Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev 2004; 30:609 - 41; http://dx.doi.org/10.1016/j.ctrv.2004.06.010; PMID: 15531395
- Sebti SM, Hamilton AD. Farnesyltransferase and geranylgeranyltransferase I inhibitors and cancer therapy: lessons from mechanism and bench-to-bedside translational studies. Oncogene 2000; 19:6584 - 93; http://dx.doi.org/10.1038/sj.onc.1204146; PMID: 11426643
- Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev 1993; 73:617 - 38; PMID: 8332640
- Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 2012; 23:2066 - 75; http://dx.doi.org/10.1091/mbc.E11-10-0884; PMID: 22496421
- Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M. Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc Natl Acad Sci U S A 1992; 89:3000 - 4; http://dx.doi.org/10.1073/pnas.89.7.3000; PMID: 1557405
- Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, Dalton M. The processing pathway of prelamin A. J Cell Sci 1994; 107:61 - 7; PMID: 8175923
- Hennekes H, Nigg EA. The role of isoprenylation in membrane attachment of nuclear lamins. A single point mutation prevents proteolytic cleavage of the lamin A precursor and confers membrane binding properties. J Cell Sci 1994; 107:1019 - 29; PMID: 8056827
- Krohne G, Waizenegger I, Höger TH. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J Cell Biol 1989; 109:2003 - 11; http://dx.doi.org/10.1083/jcb.109.5.2003; PMID: 2808518
- Lee R, Chang SY, Trinh H, Tu Y, White AC, Davies BS, et al. Genetic studies on the functional relevance of the protein prenyltransferases in skin keratinocytes. Hum Mol Genet 2010; 19:1603 - 17; http://dx.doi.org/10.1093/hmg/ddq036; PMID: 20106865
- Davies BS, Barnes RH 2nd, Tu Y, Ren S, Andres DA, Spielmann HP, et al. An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Hum Mol Genet 2010; 19:2682 - 94; http://dx.doi.org/10.1093/hmg/ddq158; PMID: 20421363
- Rahman-Roblick R, Roblick UJ, Hellman U, Conrotto P, Liu T, Becker S, et al. p53 targets identified by protein expression profiling. Proc Natl Acad Sci U S A 2007; 104:5401 - 6; http://dx.doi.org/10.1073/pnas.0700794104; PMID: 17372198
- Gerace L, Huber MD. Nuclear lamina at the crossroads of the cytoplasm and nucleus. J Struct Biol 2012; 177:24 - 31; http://dx.doi.org/10.1016/j.jsb.2011.11.007; PMID: 22126840
- Zhang YQ, Sarge KD. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol 2008; 182:35 - 9; http://dx.doi.org/10.1083/jcb.200712124; PMID: 18606848
- Gerace L, Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell 1980; 19:277 - 87; http://dx.doi.org/10.1016/0092-8674(80)90409-2; PMID: 7357605
- Adolph KW. ADPribosylation of nuclear proteins labeled with [3H]adenosine: changes during the HeLa cycle. Biochim Biophys Acta 1987; 909:222 - 30; http://dx.doi.org/10.1016/0167-4781(87)90081-9; PMID: 3040104
- Moulson CL, Go G, Gardner JM, van der Wal AC, Smitt JH, van Hagen JM, et al. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol 2005; 125:913 - 9; http://dx.doi.org/10.1111/j.0022-202X.2005.23846.x; PMID: 16297189
- Yang SH, Andres DA, Spielmann HP, Young SG, Fong LG. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J Clin Invest 2008; 118:3291 - 300; http://dx.doi.org/10.1172/JCI35876; PMID: 18769635
- Yang SH, Chang SY, Ren S, Wang Y, Andres DA, Spielmann HP, et al. Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Hum Mol Genet 2011; 20:436 - 44; http://dx.doi.org/10.1093/hmg/ddq490; PMID: 21088111
- Adjei AA, Davis JN, Erlichman C, Svingen PA, Kaufmann SH. Comparison of potential markers of farnesyltransferase inhibition. Clin Cancer Res 2000; 6:2318 - 25; PMID: 10873082
- Tamanoi F, Kato-Stankiewicz J, Jiang C, Machado I, Thapar N. Farnesylated proteins and cell cycle progression. J Cell Biochem Suppl 2001; Suppl 37 64 - 70; http://dx.doi.org/10.1002/jcb.10067; PMID: 11842430
- Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, et al. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 2006; 311:1887 - 93; http://dx.doi.org/10.1126/science.1122771; PMID: 16543417
- Yang SH, Chang SY, Tu Y, Lawson GW, Bergo MO, Fong LG, et al. Severe hepatocellular disease in mice lacking one or both CaaX prenyltransferases. J Lipid Res 2012; 53:77 - 86; http://dx.doi.org/10.1194/jlr.M021220; PMID: 22039581
- Hegele RA, Cao H, Liu DM, Costain GA, Charlton-Menys V, Rodger NW, et al. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet 2006; 79:383 - 9; http://dx.doi.org/10.1086/505885; PMID: 16826530
- Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, et al. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet 2011; 20:3537 - 44; http://dx.doi.org/10.1093/hmg/ddr266; PMID: 21659336
- Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell 2012; 148:752 - 64; http://dx.doi.org/10.1016/j.cell.2011.11.064; PMID: 22341447
- Sepp-Lorenzino L, Rosen N. A farnesyl-protein transferase inhibitor induces p21 expression and G1 block in p53 wild type tumor cells. J Biol Chem 1998; 273:20243 - 51; http://dx.doi.org/10.1074/jbc.273.32.20243; PMID: 9685373
- Wang Y, Ostlund C, Choi JC, Swayne TC, Gundersen GG, Worman HJ. Blocking farnesylation of the prelamin A variant in Hutchinson-Gilford progeria syndrome alters the distribution of A-type lamins. Nucleus 2012; 3:452 - 62; http://dx.doi.org/10.4161/nucl.21675; PMID: 22895092
- Sinensky M, Fantle K, Dalton M. An antibody which specifically recognizes prelamin A but not mature lamin A: application to detection of blocks in farnesylation-dependent protein processing. Cancer Res 1994; 54:3229 - 32; PMID: 8205544
- Dreyfuss G, Adam SA, Choi YD. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol 1984; 4:415 - 23; PMID: 6717428
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92:9363 - 7; http://dx.doi.org/10.1073/pnas.92.20.9363; PMID: 7568133
- Yuan J, Wang X, Zhang Y, Hu X, Deng X, Fei J, et al. shRNA transcribed by RNA Pol II promoter induce RNA interference in mammalian cell. Mol Biol Rep 2006; 33:43 - 9; http://dx.doi.org/10.1007/s11033-005-3965-1; PMID: 16636916