Abstract
Maintenance of nuclear architecture is crucial for gene regulation, cell proliferation and tissue development. However, during every open mitosis and meiosis, chromosomes are exposed to cytoskeletal forces until they are fully reassembled into mature nuclei. Here we discuss our recent study of nuclear assembly in Xenopus egg extracts, where we showed that the DNA binding protein Developmental pluripotency associated 2 (Dppa2) directly inhibits microtubule polymerization during nuclear formation, and that this is essential for normal nuclear shape and replication. We explore mechanisms by which microtubule dynamics could regulate nuclear formation and morphology, and discuss the importance of both spatial and temporal regulation of microtubules in this process. Moreover, expression of Dppa2 is limited to the early embryo and pluripotent tissues, and we highlight the specific demands of mitosis in these often rapidly dividing cells, in which telophase nuclear assembly must be expedited and may facilitate developmental changes in nuclear architecture.
Introduction
A diverse range of nuclear sizes, shapes and structures are observed in physiologically normal organisms, yet rarely do we understand how distinct nuclear morphologies are achieved or what functions they accomplish.Citation1,Citation2 The nuclear envelope plays a key role in shaping nuclear architecture, by providing a physical scaffold as well as multiple attachment sites to chromatin through inner nuclear membrane (INM) proteins.Citation3 The structural importance of INM proteins is exemplified by neutrophils, in which loss of lamin A promotes nuclear deformability to allow extravasation through narrow tissue spaces.Citation4 Similarly, INM proteins can organize nuclear contents, as seen in the rod photoreceptor cells of nocturnal mammals where downregulation of lamin A/C and lamin B receptor repositions heterochromatin to the center of the nucleus to reduce light scattering.Citation5 Abnormal nuclear morphology is frequently observed in cancers and many tissue dystrophies, which again is often linked to mutations in INM proteins.Citation6,Citation7 However, even when the structural components of the nucleus are intact, the nucleus is wholly dismantled during every open mitosis and meiosis, and left critically vulnerable to outside forces until its reassembly is complete.
Nuclear Reassembly Following Mitosis and Meiosis
The nuclear division cycle is driven by cell cycle stage-specific phosphorylation and regulated proteolysis.Citation8,Citation9 The final events of M phase, involving spindle disassembly, chromatin decondensation and nuclear envelope assembly (), require dephosphorylation and/or destruction of M phase effectors.Citation10 Spindle disassembly is accomplished first by extinction of cyclin-dependent kinase 1 (Cdk1) activity, which causes a global change in microtubule behavior and makes microtubules less dynamic,Citation11,Citation12 and second by degradation of spindle assembly factors such as HURP and NuSAP by the anaphase-promoting complex/cyclosome.Citation13 Chromosome decondensation requires the removal of the mitotic kinase Aurora B from chromosomes.Citation14,Citation15 Aurora B is a subunit of the heterotetrameric chromosomal passenger complex (CPC), together with its partners INCENP, Borealin (also known as Dasra) and Survivin. Importantly, kinase inactivation alone of Cdk1 or Aurora B is insufficient for the events of mitotic exit, and phosphorylation marks placed by mitotic kinases must also be removed by phosphatases.Citation16-Citation18 This is especially important for nuclear assembly, since persistent phosphorylation of INM proteins or nucleoporins inhibits assembly of the nuclear envelope and nuclear pore complexes,Citation16,Citation19-Citation22 and phosphatase activity is recruited to chromatin during telophase to promote nuclear formation.Citation22
Figure 1. Nuclear assembly during mitosis and fertilization. (A) During mitotic exit, spindle disassembly is coordinated with chromosome decondensation and nuclear envelope formation. During fertilization, completion of female meiosis II follows largely the same events, with disassembly of the meiotic spindle and assembly of the maternal pronucleus; the only major difference is that one meiotic daughter cell is extruded as a polar body (not pictured). (B) Fertilization also requires the dramatic conversion of compact sperm chromatin into decondensed spherical pronuclei. Concomitantly, astral microtubules nucleated from sperm centrosomes capture both sperm and egg pronuclei and transport them toward one another at the center of the egg. ♂ indicates sperm chromosomes and pronucleus, ♀ indicates female pronucleus. (C) Nuclear (and pronuclear) assembly results in assembly of a double nuclear membrane and nuclear pore complexes around decondensed chromatin.
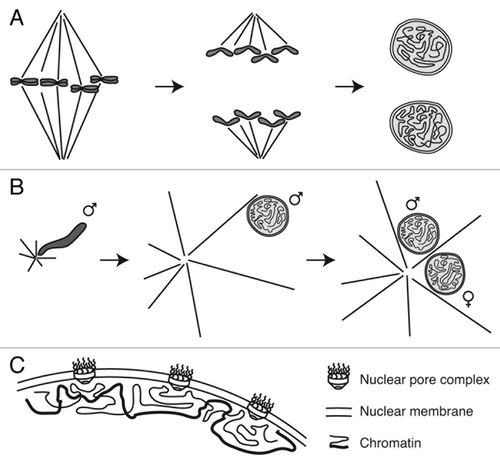
All of these events are temporally coordinated, and disruption of this ordered sequence can lead to nuclear aberrations and compromise subsequent nuclear function. Abnormal mitosis is known to cause DNA breaks and chromosome translocations,Citation23 as well as aneuploidy and proteomic imbalance.Citation24 Strikingly, incompletely segregating chromosomes can become separated from the rest of the anaphase chromosomes due to aberrant spindle attachments or motor protein dysfunction.Citation25,Citation26 If the cell proceeds to nuclear envelope assembly without correcting these errors, the lagging chromosomes may become permanently encapsulated in micronuclei.Citation25,Citation26 These micronuclei can persist over subsequent cell divisions, and have weakened nuclear envelopes that cannot support proper nuclear compartmentalization, transcription or DNA replication.Citation26,Citation27 This is thought to create DNA damage and genomic instability, underscoring the importance of correctly executing mitosis for nuclear assembly and cellular function.
Pronuclear Assembly and the Xenopus Egg Extract System
Nuclear assembly in dividing cells has much in common with the events of fertilization and pronuclear assembly. In many animals, including humans and Xenopus, eggs are arrested at metaphase of meiosis II until release upon sperm entry. Female meiosis then completes and the egg chromosomes assemble into the maternal pronucleus in a process that is largely identical to nuclear assembly following mitosis (). Sperm follow a similar though not identical fate. Sperm nuclei are extensively remodeled during spermatogenesis, involving replacement of histones with protamines, assembly of a specialized nuclear envelope with few nuclear pores and adoption of specialized nuclear shapes.Citation28 When sperm chromosomes are exposed to egg cytoplasm, these changes are reversed, and the chromosomes decompact, load histones and assemble into the spherical paternal pronucleus with a high density of nuclear pores (). Although sperm pronuclear assembly is not associated with disassembly of any spindle, sperm nuclei bring centrosomes, which nucleate a distinct microtubule structure, namely the sperm aster (). This aster quickly grows to span the length of the egg, captures both the maternal and paternal pronuclei, and transports them toward one another in preparation for zygotic mitosis.Citation29
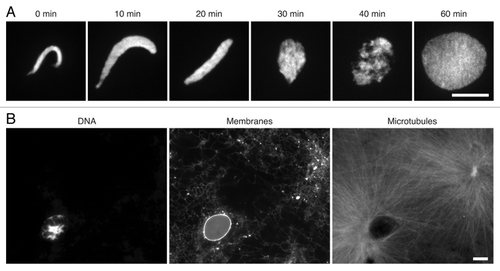
The dramatic changes observed during sperm pronuclear assembly make it a sensitive system to dissect the requirements of nuclear formation, including chromosome decondensation, membrane recruitment and nuclear pore assembly (). Cell-free extracts of Xenopus eggs robustly recapitulate this process, and have yielded many mechanistic insights into nuclear assembly.Citation30,Citation31 To mimic fertilization, sperm chromatin is added to metaphase-arrested egg extracts together with calcium. Pronuclear assembly can then be monitored as the dramatic conversion of compact, crescent shaped sperm nuclei into decondensed spheres (). At the same time, egg extracts also recapitulate the activity of sperm centrosomes,Citation29,Citation32 which rapidly assemble astral microtubules around the nascent pronucleus (). Importantly, mature sperm bring a limited complement of proteins, and the majority of chromatin-binding proteins that shape and organize the pronucleus—from histones to lamins—are loaded from the maternal pool in the egg. Specific factors can therefore be depleted from egg extracts to analyze their roles in nuclear assembly, which may otherwise be difficult in dividing cells where genetic deletions of critical proteins could be lethal.
Figure 2. Pronuclear assembly in Xenopus egg extracts. (A) Sperm chromatin was added to metaphase egg extracts, released into interphase and visualized at the indicated time points with 1 μg/ml Hoechst 33342. (B) Fully assembled pronucleus at 60 min. DNA was visualized with Hoechst 33342; nuclear and endoplasmic-reticular membrane with 1 μM CM-DiI lipophilic dye; microtubules with 0.2 μM Alexa-Fluor-488 labeled bovine tubulin. Scale bars, 10 μm.
In our recent study, we revealed that pronuclear assembly depends on finely balanced microtubule dynamics, controlled spatially and temporally by chromatin-bound regulators.Citation33 Excessive microtubule polymerization, for example induced by the drug taxol, causes assembly of distorted nuclei with irregular shapes. We identified a regulator of this process, Developmental pluripotency associated 2 (Dppa2), from a mass spectrometry-based survey of proteins bound to chromatin in Xenopus egg extracts. We showed that Dppa2 is a novel suppressor of microtubule polymerization that controls microtubules during nuclear formation. Depletion of Dppa2 from Xenopus egg extracts mimics taxol treatment, leading to enhanced sperm aster microtubule polymerization and abnormal nuclear shape with reduced lamin and nuclear pore assembly. These abnormal nuclei are also functionally compromised, showing delayed and disorganized DNA replication ().
Figure 3. Dppa2 is required for nuclear shape and organized DNA replication. (A) Nuclei assembled in mock depleted and Dppa2-depleted Xenopus egg extracts were fixed at 60 min after release into interphase. DNA was visualized with Hoechst 33342 and replication by incorporation of 1 μM fluorescein labeled dUTP. Scale bar, 10 μm. (B) Chromatin-bound Dppa2 suppresses local microtubule assembly during nuclear formation. Microtubules play both positive (“+”) and negative (“–”) roles on nuclear formation.
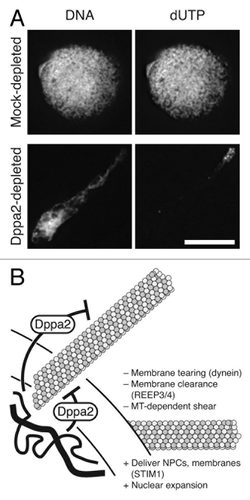
We discovered that we could rescue the effects of Dppa2 depletion by reducing microtubule assembly to normal levels. The CPC is an important promoter of microtubule polymerization around chromatin, and its subunits can be stoichiometrically depleted from egg extracts with anti-INCENP antibodies.Citation34 Co-depletion of the CPC reduces microtubule polymerization and concomitantly recues nuclear shape in Dppa2-depleted extracts.Citation33 We achieved the same effect with a low dose of nocodazole that reduces—but does not abolish—microtubule assembly.Citation33 This level of nocodazole also rescues DNA replication in Dppa2-depleted extracts. However, while higher concentrations of nocodazole continue to rescue nuclear shape, when microtubules are completely abolished nuclear size is concomitantly reduced. This is consistent with evidence that membranes and nuclear pore complexes are delivered to nascent nuclei along microtubule tracks.Citation35 Thus, microtubules play both positive and negative roles during pronuclear assembly; some microtubules are required to promote nuclear growth, but excessive polymerization leads to nuclear shape and function defects (). We confirmed that the effects of Dppa2 depletion and treatment with nocodazole or taxol are not due to altered cell cycle timing, since mitotic kinase activities are downregulated normally after these treatments, and nuclear assembly defects persist even in the absence of the spindle assembly checkpoint.Citation33
Spatial Control of Microtubule Dynamics
We showed that Dppa2 suppresses assembly of microtubules both in Xenopus egg extracts and from purified tubulin in vitro. However, Dppa2 is unique among microtubule regulators in that it is localized exclusively to chromatin.Citation33 We propose that this is essential to spatially restrict Dppa2 activity. By deleting a conserved DNA-binding domain in Dppa2, the SAP (SAF-A/B, Acinus, and PIAS) motif,Citation36 we were able to separate the DNA-binding and microtubule-inhibitory functions of Dppa2. While DNA binding is dispensable for inhibiting microtubule assembly, it is still required to promote nuclear formation. We suggest that DNA binding allows Dppa2 to suppress microtubules specifically in the vicinity of chromatin to protect the nucleus. Electron microscopy studies of telophase nuclear assembly in human cells have revealed the presence of microtubules occupying gaps in the nascent nuclear membrane.Citation37 Similarly, live imaging has demonstrated that during nuclear assembly lamin B receptor is initially recruited to chromatin at sites away from microtubule density.Citation38,Citation39 These results suggest that microtubules could physically obstruct nuclear envelope assembly. Indeed, when anaphase HeLa cells are treated with nocodazole or taxol, the timing of nuclear envelope closure is accelerated or delayed respectively.Citation40 Moreover, microtubule-dependent motor proteins may also interfere with nuclear formation, since dynein can bind and tear the nuclear envelope, a process that contributes to nuclear envelope breakdown at mitotic entry.Citation41,Citation42
In addition to presenting structural impediments to nuclear envelope assembly, microtubules may also produce signaling events that delay nuclear formation. For example, Aurora B inhibits chromosome decondensation and must be removed from chromatin at mitotic exit,Citation14 and Aurora B kinase activity is stimulated by microtubules.Citation34 Dppa2 could therefore suppress microtubule polymerization to counteract such signaling pathways. However, excess microtubule polymerization induced by taxol impedes nuclear assembly even in CPC-depleted extracts,Citation33 indicating that the effect of microtubules on nuclear assembly cannot be attributed solely to Aurora B. Localizing Dppa2 activity to chromatin may represent a mechanism for local control so as not to disturb microtubule dynamics elsewhere in the cell. This is especially important in the millimeter-scale Xenopus egg, where disparate populations of microtubules may accomplish diverse functions around the cell and thus require distinct local regulators. In our study we examined pronuclear assembly and found that it was inhibited by stabilization of sperm aster microtubules (), but remnants of mitotic spindle microtubulesCitation40 could have similar effects during nuclear formation after mitotic exit (). In both cases, we predict that the strongest perturbations of nuclear assembly would come from the behavior of microtubules in the immediate vicinity of chromatin.
Temporal Control of Microtubule Dynamics
In addition to spatial control, we discovered that nuclear assembly is uniquely sensitive to microtubule dynamics during a specific time window at the transition from M phase to interphase, when nuclear assembly is still in its early stages.Citation33 Taxol only causes nuclear defects if added during this window, and similarly abnormal nuclear shape in Dppa2-depleted extracts is only rescued by nocodazole if the drug is added during this time. There is therefore a specific requirement for regulated microtubule dynamics during early nuclear formation. We suggest that, during this time, the nascent nucleus has not yet acquired sufficient strength to resist microtubule-dependent shear forces, such as those implicated in nuclear envelope breakdown.Citation41,Citation42 Microtubule-generated forces could induce permanent deformations, which are not rescued when microtubules are abolished at later time points. Once nuclear envelope assembly is complete, mature nuclei exclude microtubulesCitation43 and become strong enough to resist external stresses. This strength may be conferred by the continuous import and assembly of structural proteins such as lamins,Citation44 although lamins may not be the only proteins involved since they are dispensable for normal cell division.Citation45
At the same time as nuclear formation begins, exit from M phase brings a global change in microtubule dynamics.Citation11,Citation12 Specifically, the frequency of microtubule catastrophe, which refers to a transition from growth to shrinkage, decreases 10-fold, and interphase microtubules typically exhibit unbounded growth in a constant direction.Citation12 This stabilization may lessen the stress that nuclei experience relative to M phase, when they are pushed and pulled by many microtubules and microtubule-associated motors in different and changing directions.
Our data support the notion that microtubules play both positive and negative roles in nuclear assembly, since taxol treatment impairs nuclear morphology but nocodazole treatment delays nuclear expansion.Citation33 Interestingly, a number of proteins, namely STIM1 and REEP3/4, can interact with both microtubules and endoplasmic reticular membranes.Citation46,Citation47 REEP3 and REEP4 are required to clear membranes away from metaphase chromosomes, likely by microtubule minus-end directed transport,Citation47 while mitotic phosphorylation of the transmembrane protein STIM1 causes it to dissociate from microtubule plus ends, preventing invasion of membranes into the spindle.Citation46 There exists therefore a physical network of connections between membranes and microtubules. As the cell division cycle unfolds, this network is dynamically tuned, for example by phosphoregulation,Citation46 so that chromosomes initially exclude membranes during spindle assembly, but switch to excluding microtubules during nuclear formation.
Nuclear Assembly in Embryonic Systems
Like its mammalian homologs, Xenopus Dppa2 is expressed exclusively in the early embryo.Citation48,Citation49 Embryonic tissues often divide rapidly; this is especially apparent in Xenopus embryos which cleave every 30 min until the mid-blastula transition.Citation50 Such short cell cycles are accomplished by eliminating gap phases, but can also involve specialized mechanisms to accelerate mitosis. For example, in early frog and zebrafish embryos, nuclear envelope assembly is initiated before chromosome segregation is complete, enclosing individual anaphase chromatids inside micronuclei known as karyomeres.Citation51,Citation52 Karyomeres are distinct from the micronuclei that result from chromosome segregation errors,Citation26,Citation27 and instead allow DNA replication to begin early and gain a head start on the next mitosis.Citation51 Dppa2 may help to facilitate such rapid nuclear formation in the early embryo, consistent with antisense morpholino-mediated knockdown experiments which showed that Dppa2 is required for early embryonic development.Citation49 However, we note that our experiments using Xenopus egg extracts most closely mimic fertilization, which takes place less rapidly; the first cell division of Xenopus takes around 80 min, from meiotic metaphase II to the first diploid metaphase,Citation53 compared with 30 min in subsequent mitoses.Citation50 Dppa2 may play a key role in ensuring proper pronuclear assembly and fertilization, which would not be apparent in morpholino experiments that cannot remove the maternal pool of Dppa2 protein stored in the egg.
We expect that other specialized mechanisms to promote rapid mitosis remain to be discovered both in embryonic cells and rapidly dividing somatic cells. Similarly, misregulated mitotic timing could contribute to disease. For example, chromatin-induced signals promote both spindle assembly and nuclear envelope formation,Citation30,Citation31,Citation54 and chromosomal gain in aneuploid cancer cells has been proposed to accelerate mitotic progression by amplifying these signals.Citation55
Additional Functions of Dppa2
The C terminus of Xenopus Dppa2, which is critical for its activity to inhibit microtubule polymerization,Citation33 does not appear to be conserved in mammals.Citation49 In our study we did not address whether homologs of Dppa2 possess the same functionality, but it is possible that microtubule inhibition is dispensable in mammalian embryos, which divide relatively slowly compared with Xenopus. Chromatin immunoprecipitation experiments suggest that murine Dppa2 functions as a transcription factor and binds the promoters of the developmental genes Nkx2–5 and Syce1.Citation56 Moreover, Dppa2 can act as a reprogramming factor in the derivation of mouse induced pluripotent stem cells.Citation57 Dppa2 genes appear therefore to be important across different species for embryonic nuclear function. Future studies are needed to investigate whether Dppa2 regulates gene expression in all organisms, whether it does so directly or indirectly, how it might interface with nuclear architecture via the lamina or nuclear pores and whether it binds specific DNA sequences or chromatin environments.
Concluding Remarks
Nuclear morphology and organization can influence cellular function both by directly controlling the physical properties of the nucleus and by indirectly influencing gene expression.Citation1,Citation2 Xenopus egg extracts offer a tractable biochemical system for investigating the mechanics of nuclear assembly, allowing depletion of essential proteins that may be difficult to remove from living cells. Our study demonstrated that tight regulation of microtubule dynamics is essential for nuclear formation and is maintained in Xenopus by Dppa2.Citation33 Depletion of Dppa2 disrupted pronuclear DNA replication, but subtler perturbations of nuclear morphology could still lead to phenotypes that manifest later in development. We anticipate that many regulators of nuclear assembly will play important roles in normal development and pathogenesis. In organisms that carry out open mitosis, every cell division affords an opportunity to reshuffle nuclear organization,Citation58 and thus a chance to reshape cell function and subsequent cell fate.
| Abbreviations: | ||
| CPC | = | chromosomal passenger complex |
| Cdk1 | = | cyclin-dependent kinase 1 |
| Dppa2 | = | developmental pluripotency associated 2 |
| INM | = | inner nuclear membrane |
| SAP | = | SAF-A/B, Acinus, and PIAS |
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We thank David Wynne, Sozanne Solmaz, and members of the Funabiki lab for discussions. Images were acquired on a DeltaVision widefield microscope at The Rockefeller University Bio-Imaging Resource Center. This work was approved by The Rockefeller University Institutional Animal Care and Use Committee and supported by the National Institutes of Health (R01 GM075249/NIGMS).
References
- Edens LJ, White KH, Jevtic P, Li X, Levy DL. Nuclear size regulation: from single cells to development and disease. Trends Cell Biol 2013; 23:151 - 9; http://dx.doi.org/10.1016/j.tcb.2012.11.004; PMID: 23277088
- Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol 2013; 23:R1113 - 21; http://dx.doi.org/10.1016/j.cub.2013.11.009; PMID: 24355792
- Rothballer A, Kutay U. SnapShot: The nuclear envelope I. Cell 2012; 150:868 - e1, e1; http://dx.doi.org/10.1016/j.cell.2012.07.024; PMID: 22901815
- Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem 2013; 288:8610 - 8; http://dx.doi.org/10.1074/jbc.M112.441535; PMID: 23355469
- Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013; 152:584 - 98; http://dx.doi.org/10.1016/j.cell.2013.01.009; PMID: 23374351
- Schirmer EC, Florens L, Guan T, Yates JR 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 2003; 301:1380 - 2; http://dx.doi.org/10.1126/science.1088176; PMID: 12958361
- Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harb Perspect Biol 2010; 2:a000547; http://dx.doi.org/10.1101/cshperspect.a000547; PMID: 20826548
- Funabiki H, Wynne DJ. Making an effective switch at the kinetochore by phosphorylation and dephosphorylation. Chromosoma 2013; 122:135 - 58; http://dx.doi.org/10.1007/s00412-013-0401-5; PMID: 23512483
- Teixeira LK, Reed SI. Ubiquitin ligases and cell cycle control. Annu Rev Biochem 2013; 82:387 - 414; http://dx.doi.org/10.1146/annurev-biochem-060410-105307; PMID: 23495935
- Schooley A, Vollmer B, Antonin W. Building a nuclear envelope at the end of mitosis: coordinating membrane reorganization, nuclear pore complex assembly, and chromatin de-condensation. Chromosoma 2012; 121:539 - 54; http://dx.doi.org/10.1007/s00412-012-0388-3; PMID: 23104094
- Verde F, Labbé JC, Dorée M, Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature 1990; 343:233 - 8; http://dx.doi.org/10.1038/343233a0; PMID: 2405278
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell 1990; 62:579 - 89; http://dx.doi.org/10.1016/0092-8674(90)90022-7; PMID: 2379239
- Song L, Craney A, Rape M. Microtubule-dependent regulation of mitotic protein degradation. Mol Cell 2014; 53:179 - 92; http://dx.doi.org/10.1016/j.molcel.2013.12.022; PMID: 24462202
- Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature 2007; 450:1258 - 62; http://dx.doi.org/10.1038/nature06388; PMID: 18097415
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 2010; 330:235 - 9; http://dx.doi.org/10.1126/science.1189505; PMID: 20705815
- Schmitz MHA, Held M, Janssens V, Hutchins JRA, Hudecz O, Ivanova E, Goris J, Trinkle-Mulcahy L, Lamond AI, Poser I, et al. Live-cell imaging RNAi screen identifies PP2A-B55α and importin-β1 as key mitotic exit regulators in human cells. Nat Cell Biol 2010; 12:886 - 93; http://dx.doi.org/10.1038/ncb2092; PMID: 20711181
- Qian J, Lesage B, Beullens M, Van Eynde A, Bollen M. PP1/Repo-man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal aurora B targeting. Curr Biol 2011; 21:766 - 73; http://dx.doi.org/10.1016/j.cub.2011.03.047; PMID: 21514157
- Vagnarelli P, Ribeiro S, Sennels L, Sanchez-Pulido L, de Lima Alves F, Verheyen T, Kelly DA, Ponting CP, Rappsilber J, Earnshaw WC. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev Cell 2011; 21:328 - 42; http://dx.doi.org/10.1016/j.devcel.2011.06.020; PMID: 21820363
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 1990; 61:579 - 89; http://dx.doi.org/10.1016/0092-8674(90)90470-Y; PMID: 2344612
- Peter M, Nakagawa J, Dorée M, Labbé J-C, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 1990; 61:591 - 602; http://dx.doi.org/10.1016/0092-8674(90)90471-P; PMID: 2188731
- Laurell E, Beck K, Krupina K, Theerthagiri G, Bodenmiller B, Horvath P, Aebersold R, Antonin W, Kutay U. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell 2011; 144:539 - 50; http://dx.doi.org/10.1016/j.cell.2011.01.012; PMID: 21335236
- Asencio C, Davidson IF, Santarella-Mellwig R, Ly-Hartig TBN, Mall M, Wallenfang MR, Mattaj IW, Gorjánácz M. Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell 2012; 150:122 - 35; http://dx.doi.org/10.1016/j.cell.2012.04.043; PMID: 22770216
- Ganem NJ, Pellman D. Linking abnormal mitosis to the acquisition of DNA damage. J Cell Biol 2012; 199:871 - 81; http://dx.doi.org/10.1083/jcb.201210040; PMID: 23229895
- Tang Y-C, Amon A. Gene copy-number alterations: a cost-benefit analysis. Cell 2013; 152:394 - 405; http://dx.doi.org/10.1016/j.cell.2012.11.043; PMID: 23374337
- Ohsugi M, Adachi K, Horai R, Kakuta S, Sudo K, Kotaki H, Tokai-Nishizumi N, Sagara H, Iwakura Y, Yamamoto T. Kid-mediated chromosome compaction ensures proper nuclear envelope formation. Cell 2008; 132:771 - 82; http://dx.doi.org/10.1016/j.cell.2008.01.029; PMID: 18329364
- Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012; 482:53 - 8; http://dx.doi.org/10.1038/nature10802; PMID: 22258507
- Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013; 154:47 - 60; http://dx.doi.org/10.1016/j.cell.2013.06.007; PMID: 23827674
- Wright SJ. Sperm nuclear activation during fertilization. Curr Top Dev Biol 1999; 46:133 - 78; http://dx.doi.org/10.1016/S0070-2153(08)60328-2; PMID: 10417879
- Mitchison T, Wühr M, Nguyen P, Ishihara K, Groen A, Field CM. Growth, interaction, and positioning of microtubule asters in extremely large vertebrate embryo cells. Cytoskeleton (Hoboken) 2012; 69:738 - 50; http://dx.doi.org/10.1002/cm.21050; PMID: 22786885
- Lohka MJ, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science 1983; 220:719 - 21; http://dx.doi.org/10.1126/science.6601299; PMID: 6601299
- Forbes DJ, Kirschner MW, Newport JW. Spontaneous formation of nucleus-like structures around bacteriophage DNA microinjected into Xenopus eggs. Cell 1983; 34:13 - 23; http://dx.doi.org/10.1016/0092-8674(83)90132-0; PMID: 6224569
- Tsai M-Y, Zheng Y. Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr Biol 2005; 15:2156 - 63; http://dx.doi.org/10.1016/j.cub.2005.10.054; PMID: 16332542
- Xue JZ, Woo EM, Postow L, Chait BT, Funabiki H. Chromatin-bound Xenopus Dppa2 shapes the nucleus by locally inhibiting microtubule assembly. Dev Cell 2013; 27:47 - 59; http://dx.doi.org/10.1016/j.devcel.2013.08.002; PMID: 24075807
- Tseng BS, Tan L, Kapoor TM, Funabiki H. Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Dev Cell 2010; 18:903 - 12; http://dx.doi.org/10.1016/j.devcel.2010.05.018; PMID: 20627073
- Sutovsky P, Simerly C, Hewitson L, Schatten G. Assembly of nuclear pore complexes and annulate lamellae promotes normal pronuclear development in fertilized mammalian oocytes. J Cell Sci 1998; 111:2841 - 54; PMID: 9730977
- Aravind L, Koonin EV. SAP - a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci 2000; 25:112 - 4; http://dx.doi.org/10.1016/S0968-0004(99)01537-6; PMID: 10694879
- Haraguchi T, Kojidani T, Koujin T, Shimi T, Osakada H, Mori C, Yamamoto A, Hiraoka Y. Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J Cell Sci 2008; 121:2540 - 54; http://dx.doi.org/10.1242/jcs.033597; PMID: 18628300
- Chaudhary N, Courvalin JC. Stepwise reassembly of the nuclear envelope at the end of mitosis. J Cell Biol 1993; 122:295 - 306; http://dx.doi.org/10.1083/jcb.122.2.295; PMID: 8391536
- Gerlich D, Beaudouin J, Gebhard M, Ellenberg J, Eils R. Four-dimensional imaging and quantitative reconstruction to analyse complex spatiotemporal processes in live cells. Nat Cell Biol 2001; 3:852 - 5; http://dx.doi.org/10.1038/ncb0901-852; PMID: 11533667
- Lu L, Ladinsky MS, Kirchhausen T. Formation of the postmitotic nuclear envelope from extended ER cisternae precedes nuclear pore assembly. J Cell Biol 2011; 194:425 - 40; http://dx.doi.org/10.1083/jcb.201012063; PMID: 21825076
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 2002; 108:83 - 96; http://dx.doi.org/10.1016/S0092-8674(01)00627-4; PMID: 11792323
- Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 2002; 108:97 - 107; http://dx.doi.org/10.1016/S0092-8674(01)00628-6; PMID: 11792324
- Akoumianaki T, Kardassis D, Polioudaki H, Georgatos SD, Theodoropoulos PA. Nucleocytoplasmic shuttling of soluble tubulin in mammalian cells. J Cell Sci 2009; 122:1111 - 8; http://dx.doi.org/10.1242/jcs.043034; PMID: 19299461
- Levy DL, Heald R. Nuclear size is regulated by importin α and Ntf2 in Xenopus.. Cell 2010; 143:288 - 98; http://dx.doi.org/10.1016/j.cell.2010.09.012; PMID: 20946986
- Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan C-M, Gaiano N, Ko MSH, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 2011; 334:1706 - 10; http://dx.doi.org/10.1126/science.1211222; PMID: 22116031
- Smyth JT, Beg AM, Wu S, Putney JW Jr., Rusan NM. Phosphoregulation of STIM1 leads to exclusion of the endoplasmic reticulum from the mitotic spindle. Curr Biol 2012; 22:1487 - 93; http://dx.doi.org/10.1016/j.cub.2012.05.057; PMID: 22748319
- Schlaitz AL, Thompson J, Wong CC, Yates JR 3rd, Heald R. REEP3/4 ensure endoplasmic reticulum clearance from metaphase chromatin and proper nuclear envelope architecture. Dev Cell 2013; 26:315 - 23; http://dx.doi.org/10.1016/j.devcel.2013.06.016; PMID: 23911198
- Bortvin A, Eggan K, Skaletsky H, Akutsu H, Berry DL, Yanagimachi R, Page DC, Jaenisch R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 2003; 130:1673 - 80; http://dx.doi.org/10.1242/dev.00366; PMID: 12620990
- Siegel D, Schuff M, Oswald F, Cao Y, Knöchel W. Functional dissection of XDppa2/4 structural domains in Xenopus development. Mech Dev 2009; 126:974 - 89; http://dx.doi.org/10.1016/j.mod.2009.09.007; PMID: 19772919
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 1982; 30:675 - 86; http://dx.doi.org/10.1016/0092-8674(82)90272-0; PMID: 6183003
- Lemaitre J-M, Géraud G, Méchali M. Dynamics of the genome during early Xenopus laevis development: karyomeres as independent units of replication. J Cell Biol 1998; 142:1159 - 66; http://dx.doi.org/10.1083/jcb.142.5.1159; PMID: 9732278
- Abrams EW, Zhang H, Marlow FL, Kapp L, Lu S, Mullins MC. Dynamic assembly of brambleberry mediates nuclear envelope fusion during early development. Cell 2012; 150:521 - 32; http://dx.doi.org/10.1016/j.cell.2012.05.048; PMID: 22863006
- Wühr M, Tan ES, Parker SK, Detrich HW 3rd, Mitchison TJ. A model for cleavage plane determination in early amphibian and fish embryos. Curr Biol 2010; 20:2040 - 5; http://dx.doi.org/10.1016/j.cub.2010.10.024; PMID: 21055946
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 1996; 382:420 - 5; http://dx.doi.org/10.1038/382420a0; PMID: 8684481
- Hasegawa K, Ryu SJ, Kaláb P. Chromosomal gain promotes formation of a steep RanGTP gradient that drives mitosis in aneuploid cells. J Cell Biol 2013; 200:151 - 61; http://dx.doi.org/10.1083/jcb.201206142; PMID: 23319601
- Nakamura T, Nakagawa M, Ichisaka T, Shiota A, Yamanaka S. Essential roles of ECAT15-2/Dppa2 in functional lung development. Mol Cell Biol 2011; 31:4366 - 78; http://dx.doi.org/10.1128/MCB.05701-11; PMID: 21896782
- Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K, Klemm SL, van Oudenaarden A, Jaenisch R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 2012; 150:1209 - 22; http://dx.doi.org/10.1016/j.cell.2012.08.023; PMID: 22980981
- Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA, van Steensel B. Single-cell dynamics of genome-nuclear lamina interactions. Cell 2013; 153:178 - 92; http://dx.doi.org/10.1016/j.cell.2013.02.028; PMID: 23523135
