Abstract
We discuss our recent findings on the increase, in chronic myeloid leukemia patients treated with imatinib, of B1 lymphocytes producing IgM anti-O-linked sugars expressed by leukemic cells, paralleled by increased B-stimulating cytokines. We propose that one important effect of imatinib treatment is due to the remodelling of bone marrow microenvironment.
In the last years, tyrosine kinases have become the major targets of anti-cancer drugs. In particular, imatinib mesylate, an ATP competitive inhibitor of the constitutively activated ABL1 tyrosine kinase of the BCR-ABL oncoprotein, is approved for the treatment of chronic myelogenous leukemia (CML) in chronic phase; recent updates of the phase 3 International Randomized Study of Interferon and STI571 (IRIS) trial have confirmed both long-term efficacy and safety of the treatment, with an estimated overall survival rate of 86%.Citation1 However, resistance to imatinib can occur, or develop during treatment, leading to the failure of the therapy and highlighting the need of early markers of clinical response in order to address alternative treatments. Of course, a deeper understanding of the mechanisms underlying the function of the drug would be of help. Resistance is usually due to point mutations in the BCR-ABL ATP-binding site, that impede the binding of imatinib.Citation1,Citation2 Nevertheless, other mechanisms have been reported, such as BCR-ABL gene amplification, aberrations in other oncogenic signaling pathways, and the persistence of leukemic stem cells.Citation2 Extrinsic factors have also been hypothesized, including multidrug resistance and microenvironmental factors,Citation2 pointing to the possibility that imatinib action is not limited to the leukemic cell population. There is increasing evidence that the response to imatinib mesylate is also linked to the immune system with production of antineoplastic cytokines and activation of immune cell function by creating a bridge between innate and adaptive immunity.Citation3
In a recent paper,Citation4 we reported that in CML patients responding to imatinib treatment clinical and cytogenetic response is paralleled or preceded by early cytological, phenotypic and molecular changes in the bone marrow, involving the B cell compartment, that are not detectable in non responder patients. In particular, a raise in CD20+ B lymphocytes, coexpressing CD5 and bearing surface (s)IgM, is evidenced as early as 3 mo after the beginning of therapy and persists up to 6–9 mo. This population of cells is antibody-secreting, as an increase of IgM is measured in the bone marrow plasma of responder patients. Of note, this IgM fraction contains antibodies which are reactive with O-linked olygosaccharides expressed by leukemic cells and capable to kill them by a complement-independent mechanism. Such antibodies, described in the sera of solid cancer patients, resemble the so called “natural antibodies,” that are indeed produced by CD5+ B lymphocytes, also known as B1 lymphocytes, and in healthy donors mainly involved in anti-bacterial responses.Citation5,Citation6
Thus, administration of imatinib mesylate can actually contribute to an “immunologic bridge,” that functions exploiting natural antibodies to activate an anti-tumor reaction. How does it happen and what is the mechanism that leads to the stimulation of B1 cells? In the bone marrow of responder patients, the production of the stromal-derived factor-1 (SDF-1) and of the B lymphocyte activating factor of the tumor necrosis factor family (BAFF), both involved in normal B cell development and maturation,Citation7 is induced by imatinib, at variance with non responder patients. Thus, imatinib seems to be capable of modulating the bone marrow microenvironment leading to conditions favorable to B cell differentiation. It is tempting to speculate that imatinib treatment is able to “reset” the bone marrow microenvironment, involving stromal and/or endothelial cells possibly responsible for SDF-1 and BAFF production, that, in turn, would enhance the B cell compartment. This might be due to the therapeutic effects exerted by imatinib on the myeloid malignant progenitors,Citation8 that would be substituted by normal precursors, capable of repopulating the bone marrow with normal stromal as well as normal myeloid cells. This hypothesis seems to be also supported by our finding that in the bone marrow of responder patients, transcription of the bone morphogenetic proteins (BMP)2 and BMP7, both related to SDF-1 synthesis, are increased. Effects of imatinib on the osteoblastogenesis and bone marrow remodelling, mediated by BMP2, have been recently reported;Citation9 moreover, BAFF synthesis and release by normal myeloid cells has been described.Citation9 Also, SDF-1 has been shown to block colony forming units by both healthy and CML CD34+ cells.Citation10 Although it remains to be defined the contribution given in vivo by the CD20+CD5+ IgM-secreting B cells population observed in responder patients to the clearance of leukemic cells, they would represent the enhancement of B cell maturation and function in a restored bone marrow, as an early sign of the response to the treatment.
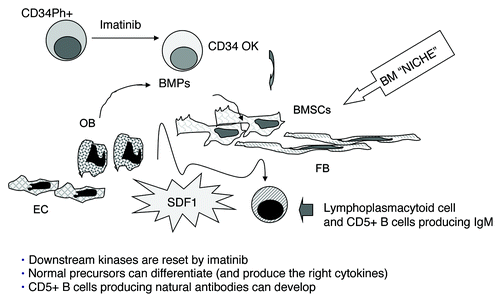
We conclude that imatinib mesylate significantly contributes to the renewal of the whole bone marrow microenvironment, due to a rescue of healthy progenitors, capable of give rise to a normal stromal compartment, composed of stromal cells, fibroblasts, osteoblasts and endothelial cells; this restored microenvironment would lead to the development of the observed B cell population producing tumor-reactive IgM, through the enhanced production of BMP, BAFF and SDF-1 (). These changes are indeed documented in the bone marrow and sera of responder patients and transiently detectable for a period of up to 6–9 mo, by which time the resetting process would be concluded, as proved by the stability of the clinical, cytogenetic and molecular response. As a statistically significant correlation to the clinical, cytogenetic and molecular response was found, we propose that the detection of IgM+ B cells and the measurement of cytokines in the bone marrow before and during treatment may contribute to the early identification of responder and non responder patients, and help in the choice and/or design of alternative therapeutic strategies.
Figure 1. Model for an additional mechanism of action of imatinib mesylate. Imatinib mesylate would contribute to the renewal of the whole bone marrow microenvironment, due to the “normalization” of hematopoietic precursors, able to give rise to healthy bone marrow stromal cells (BMSC), fibroblasts (FB), osteoblasts (OB) and endothelial cells (EC), thus rebuilding the BM “niche.” Normal production of bone morphogenetic proteins (BMP), B lymphocyte activating factor of the tumor necrosis factor family (BAFF) and stromal derived factor-1 (SDF-1) by normal BMSC, FB, EC, would remodel the microenvironment and participate into the development of the B cell population producing tumor-reactive IgM.
References
- Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukaemia. Leukemia 2009; 23:1054 - 61; http://dx.doi.org/10.1038/leu.2009.38; PMID: 19282833
- Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol 2007; 8:1018 - 29; http://dx.doi.org/10.1016/S1470-2045(07)70342-X; PMID: 17976612
- Smyth MJ. Imatinib Mesylate – Uncovering a fast track to adaptive immunity. N Engl J Med 2006; 354:2282 - 4; http://dx.doi.org/10.1056/NEJMcibr061878; PMID: 16723621
- Catellani S, Pierri I, Gobbi M, Poggi A, Zocchi MR. Imatinib treatment induces CD5+ B lymphocytes and IgM natural antibodies with anti-leukemic reactivity in patients with chronic myelogenous leukemia. PLoS One 2011. PLoS ONE 2011; 6:e18925; http://dx.doi.org/10.1371/journal.pone.0018925; PMID: 21533122
- Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol 2001; 13:195 - 201; http://dx.doi.org/10.1016/S0952-7915(00)00204-1; PMID: 11228413
- Schwartz-Albiez R, Laban S, Eichmueller S, Kirschfink M. Cytotoxic natural antibodies against human tumours: an option for anti-cancer immunotherapy?. Autoimmun Rev 2008; 7:491 - 5; http://dx.doi.org/10.1016/j.autrev.2008.03.012; PMID: 18558368
- Schaumann DH, Tuischer J, Ebell W, Manz RA, Lauster R. VCAM-1 positive stromal cells from human bone marrow producing cytokines for B lineage progenitors and for plasma cells: SDF-1, flt3L and BAFF. Mol Immunol 2007; 44:1606 - 12; http://dx.doi.org/10.1016/j.molimm.2006.08.021; PMID: 17067679
- Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ, Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukaemia through reversal of abnormally increased proliferation. Blood 2002; 99:3792 - 800; http://dx.doi.org/10.1182/blood.V99.10.3792; PMID: 11986238
- Tibullo D, Giallongo C, La Cava P, Beretta S, Stagno F, Chiarenza A, et al. Effects of imatinib mesylate in osteoblastogenesis. Exp Hematol 2009; 37:461 - 8; http://dx.doi.org/10.1016/j.exphem.2008.12.008; PMID: 19302920
- Dürig J, Rosenthal C, Elmaagacli A, Heyworth C, Halfmeyer K, Kasper C, et al. Biological effects of stroma-derived factor-1 alpha on normal and CML CD34+ haemopoietic cells. Leukemia 2000; 14:1652 - 60; http://dx.doi.org/10.1038/sj.leu.2401875; PMID: 10995013