Abstract
Platinum-based anticancer drugs enhance the immunostimulatory potential of DCs and decrease the immunosuppressive capacity of tumor cells. This immunomodulatory ability is based on the inhibition of STAT6-mediated expression of co-inhibitory molecule PD-L2 and opens up the possibility of using these drugs in combination with other immunostimulatory compounds.
Platinum-based chemotherapeutics are one of the cornerstones of contemporary cancer treatment, showing clinical efficacy against many types of cancers. Their function is based on the ability to covalently bind DNA, forming platinum-DNA adducts. Platinum-DNA adduct formation activates DNA damage recognition and repair pathways and ultimately leads to apoptosis, explaining the powerful cytotoxic properties.
Until recently these and other anticancer drugs were believed to have an immunosuppressive effect due to their myelosuppressive nature. However, recent findings point to the intriguing possibility that the opposite may be true. At least part of the clinical effect of platinum anticancer drugs may be attributed to modulation of the immune system by induction of immunogenic cell death or sensitization of tumor cells for T cell killing.Citation1-Citation3
Previous studies mainly focused on the effect of platinum drugs on tumor cells, but the tumor microenvironment also encompasses immune cells such as dendritic cells (DCs). The effect of platinum drugs on immune cells has not been studied in detail. In a recent study we investigated the effect of platinum drugs on DC functionality.Citation4
We exposed monocyte-derived DCs to clinically relevant concentrations of different chemotherapeutic drugs during their maturation and subsequently assessed their T cell stimulatory capacity using both allogeneic and antigen-specific in vitro assays. We found that only the platinum-based chemotherapeutics augmented the capacity of DCs to induce antigen-specific T cell proliferation. Furthermore, these T cells displayed increased production of IFNγ and IL-2 upon stimulation. The increased T cell stimulatory capacity was not caused by increased expression of co-stimulatory molecules or increased secretion of pro-inflammatory cytokines, but by downregulation of inhibitory molecules Programmed Death Ligand (PD-L) 1 and particularly PD-L2 on the DCs.
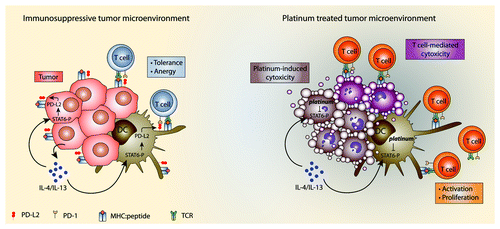
PD-L1 and 2 are ligands of PD-1 on T cells and induce tolerance and anergy.Citation5 PD-L2 expression is regulated by the IL-4/Signal transducer and activator of transcription 6 (STAT6) signaling pathway. Others have shown that IL-4 and IL-13 are abundantly present in the tumor microenvironment resulting in STAT6 activation.Citation6 We found that platinum chemotherapeutics reversed the IL-4 induced phosphorylation of STAT6 in DCs as detected by westernblot. In accordance, siRNA mediated knockdown of STAT6 in DCs decreased the platinum-induced downregulation of PD-L2 and abolished the ability of platinum drugs to enhance T cell proliferation, showing that this effect is caused by inhibition of STAT6. These results show that platinum drugs can modulate immune responses by relieving inhibitory mechanisms () and represent a novel immune-modulating function of platinum chemotherapeutics.
Figure 1. Immune modulation by platinum chemotherapeutics. (A) Immunosuppressive tumor microenvironment. IL-4/IL-13 production by tumor cells and immune cells (not shown) leads to STAT6 phosphorylation in DCs and tumor cells. STAT6 phosphoylation leads to upregulation of PD-L2 expression resulting in immune evasion by induction of T cell tolerance and anergy. (B) Platinum treated tumor microenvironment. Platinum chemotherapeutics have a direct cytotoxic effect and inhibit STAT6 phosphorylation leading to a downregulation of PD-L2 expression. Decreased PD-L2 expression leads to increased activation and proliferation of T cells by DCs and enhanced recognition of tumor cells by T cells.
Not only antigen-presenting but also tumor cells express PD-L1 and 2. This results in evasion of T cell-mediated killing and is correlated with a poor prognosis.Citation7 Thus, we hypothesized that platinum chemotherapeutics could downregulate PD-L2 on tumor cells. Indeed, also in tumor cell lines we found that treatment with platinum drugs resulted in dephosphorylation of STAT6 and subsequent downregulation of PD-L2 (but not PD-L1) and enhanced recognition by tumor-specific cytotoxic T cell clones ().
To determine the possible clinical importance of these in vitro findings we performed a retrospective clinical study. In this study, we assessed the recurrence-free survival of patients with squamous cell head and neck cancer, who had been treated with either cisplatin in combination with radiotherapy or radiotherapy alone and correlated it with the expression of STAT6 by the tumor cells. Patients with STAT6-expressing tumors had a significantly better recurrence-free survival when they had been treated with cisplatin in combination with radiotherapy. Notably, this effect was not seen in the patients that had been treated with radiotherapy alone. In fact, there was a clear trend for a lower recurrence-free survival in this treatment group for patients with STAT6-expressing tumors. These findings, which are in accordance with previous studies in colon cancer,Citation8 indicate that tumor STAT6 expression in itself results in a poor prognosis due to its immune-evasive potential. However, because platinum compounds inactivate this negative pathway, tumor STAT6 expression results in a better prognosis when treated with this group of drugs.
Whether STAT6 inactivation has only immunologic consequences still remains an unanswered question, since other studies suggest a role for STAT6 in enhancing tumor cell proliferation and invasion.Citation9 More studies on the effects of platinum-induced STAT6 inactivation on tumor cell biology are therefore warranted. In addition, to truly assess the prognostic and predictive value of STAT6-expression in platinum-sensitive tumors, prospective clinical studies are needed.
Combining the recent data, a picture emerges wherein platinum chemotherapeutics transform the immunosuppressive tumor microenvironment into an immunostimulatory site. A number of steps can be distinguished in this process (1) induction of immunogenic cell death which leads to the release of tumor antigens and results in DC activation,Citation1 (2) enhancing the T cell stimulatory capacity of DCs through downregulation of PD-L1 and 2 4 and (3) sensitization of tumor cells for T cell mediated killing by expression of mannose-6 receptor and downregulation of PD-L2.Citation3,Citation4
In conclusion, from these studies it is evident that platinum chemotherapeutics not only have a direct cytotoxic effect but also have significant immune modulating effects. Since the effector cells of the immune system appear to be unaffected as demonstrated in patients receiving oxaliplatin or cisplatin,Citation10 it is tempting to speculate that the anti-tumor efficacy could be further increased if the immunological effect of platinum drugs could be exploited and augmented by combining it with other immunotherapeutic approaches. Since several novel immunotherapies have been recently approvedCitation2 these findings could be readily translated to the clinic.
| Abbreviations: | ||
| DC | = | dendritic cell |
| PD-L | = | Programmed Death Ligand |
| STAT6 | = | Signal transducer and activator of transcription 6 |
Acknowledgments
The research described here is supported by grants from the Netherlands Organization for Scientific Research (grant 920-03-250), the Sacha Swarttouw-Hijmans Foundation, The Vanderes Foundation, and the Dutch Cancer Society.
References
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050 - 9; http://dx.doi.org/10.1038/nm1622; PMID: 17704786
- Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy - revisited. Nat Rev Drug Discov 2011; 10:591 - 600; http://dx.doi.org/10.1038/nrd3500; PMID: 21804596
- Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010; 120:1111 - 24; http://dx.doi.org/10.1172/JCI40269; PMID: 20234093
- Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011; 121:3100 - 8; http://dx.doi.org/10.1172/JCI43656; PMID: 21765211
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677 - 704; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090331; PMID: 18173375
- Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med 2007; 204:1037 - 47; http://dx.doi.org/10.1084/jem.20061120; PMID: 17438063
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11:2947 - 53; http://dx.doi.org/10.1158/1078-0432.CCR-04-1469; PMID: 15837746
- Wang CG, Ye YJ, Yuan J, Liu FF, Zhang H, Wang S. EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis. World J Gastroenterol 2010; 16:2421 - 7; http://dx.doi.org/10.3748/wjg.v16.i19.2421; PMID: 20480530
- Merk BC, Owens JL, Lopes MB, Silva CM, Hussaini IM. STAT6 expression in glioblastoma promotes invasive growth. BMC Cancer 2011; 11:184; http://dx.doi.org/10.1186/1471-2407-11-184; PMID: 21595984
- Lesterhuis WJ, de Vries IJ, Aarntzen EA, de Boer A, Scharenborg NM, van de Rakt M, et al. A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients. Br J Cancer 2010; 103:1415 - 21; http://dx.doi.org/10.1038/sj.bjc.6605935; PMID: 20924373