Abstract
NK cells, which contribute to tumor immunosurveillance, are present in the microenvironment of Non-Small-Cell Lung Carcinoma. However, they display strongly altered phenotype with decreased expression of NKp30, NKp80, DNAM-1, CD16 and ILT2, and impaired cytotoxic functions. The possible mechanisms leading to these defects are discussed.
Introduction
Natural killer (NK) cells are innate immune effector cells capable of recognizing and killing tumor cells through the release of cytotoxic enzymes and cytokines. The importance of NK cell-mediated tumor immunosurveillance has been underlined in NK cell-deficient mouse models but limited informations are available in humans. Several studies conducted on intratumoral NK cells from human solid tumors indicate that their phenotype and function are largely altered.Citation1-Citation4
In NSCLC, distinct immune cells infiltrate both the tumor nest and the stroma. Among these cells, the density of mature dendritic cells and CD3+ T cells in Tumor induced-Bronchus-Associated Lymphoid Tissue is associated with a favorable clinical outcome.Citation5 Our recent study performed on 86 early stages NSCLC patients revealed that NKp46+ cells infiltrate the tumor environment.Citation6
NK Cells are Modulated by the NSCLC Microenvironment
We conducted a series of prospective studies to determine the phenotype of intratumoral NK cells, and compared it with the peripheral blood, normal lung, emphysema or bronchectasis tissue. Among the 17 NK receptors studied, the expression of NKp30, NKp80, DNAM-1, CD16 and ILT2 was specifically decreased on intratumoral NK cells and the expression of CD69 and NKp44 activation markers was increased. Unsupervised hierarchical clustering of cell surface marker expression revealed two groups of patients, with one group in which the expression of the five receptors was highly downregulated. We did not yet get the clinical follow up of the patients, but we can speculate that the impact on NK cells on clinical outcome of the patients, if any, should be more dependent on NK cell phenotype than on the NK cell density in the tumor microenvironment. With respect to this hypothesis, we did not find that the density of NK cells either in the invasive margin or in the stroma of the tumor was associated with good or bad clinical outcome of patients.Citation6
According to this altered phenotype, NK cells functions were also found altered, with impaired degranulation capacities and IFNγ secretion, which could not be reversed following in vitro stimulation with IL-2. These impaired functions are likely due to a lack of stimulation of NK cells by tumor cells through activating NK receptors that were found decreased. Moreover, the decrease of CD16 expression should also impair the capacity of NK cells to mediate cell killing by ADCC.
Coculture experiments of NK cells from blood of healthy donors with NSCLC cell lines induced a downregulation of the same activating receptors, indicating that membrane or soluble factors expressed by tumor cells affect the NK cell phenotype. Among these factors, we identified TGFβ as a downregulation factor for NKp30 and NKp80.Citation6
Thus, our study suggests that phenotype and cytotoxic functions of NK cells is largely suppressed in the tumor microenvironment by a number of distinct effectors and secreted factors.
Possible Causes of NK Cells Modulation in the Tumor Microenvironment
Increasing numbers of human studies reveal that the phenotype and the functions of NK cells are altered in the tumor microenvironment. We speculate that these alterations can be related to several mechanisms, including downregulation of receptor expression, shedding of the receptors (), or induction of a particular differentiation program of NK cells.
Figure 1. Possible mechanisms leading to intratumoral NK cell alterations: NK cells infiltrating lung carcinoma display altered expression of NKp30, NKp80, DNAM-1 and CD16 and impaired capacities of CD107 expression and IFNγ secretion. Tumor cells produce soluble molecules such as IDO, PGE2, TGBβ and/or express membrane molecules (NK ligands) that can downregulate or shed receptors at the surface of NK cells. NK cells can also be inhibited by TGFβ produced by regulatory T cells, that are present in the NSCLC tumor microenvironment. In addition, intratumoral NK display impaired IFNγ secretion that can cause inefficient DC maturation.
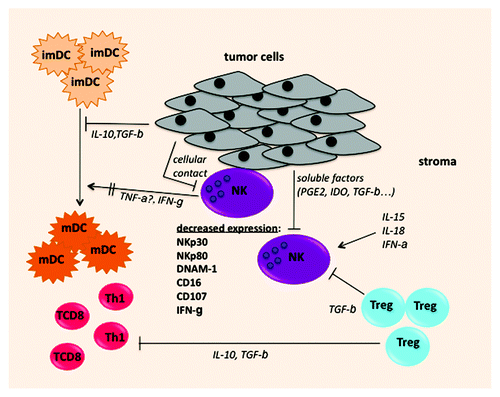
First, the modification of the phenotype and the function of NK cells can be altered in the immunosuppressive tumor microenvironment due to the high expression of membrane or soluble factors such as TGFβ, PGE2 (prostaglandin E2), LGALS3 (Galectin 3) or IDO.Citation3 In NSCLC, we observed a downregulation of NKp30, NKp80, ILT2 and DNAM1 on NK cells after a coculture with lung tumor cells, and showed that this downregulation was dependent to cell-to-cell contact and/or TGFβ. Soluble ligands for activating receptors may also downregulate NK receptors as it has been observed for sMICA/B and NKG2D. Second, the NK cells can be exhausted after tumor cells recognition and activation. It has been demonstrated that a prolonged contact of NK and tumor cells can result in the downregulation of NK receptors, such as DNAM-1, and decreased cytotoxic activity.Citation2 In some patients the tumor cells express the NKG2D, DNAM-1, as well as NCR ligands which can interact with their cognate receptors expressed by intratumoral NK cells.Citation6 Finally, we also speculate that NK cells after being recruited in the tumor site, sustain a particular developmental program of differentiation. The comparison of the gene expression profiles of NK subpopulations from the peripheral blood and from the tumor may help to decipher mechanisms leading to this particular phenotype.
Intratumoral NK Cells: Good or Bad for the Patient ?
In contrast to components of the adaptive immune response such as mature DC, or Th1 and CD8 T cells,Citation5,Citation7 and in accordance with their intratumoral NK cell phenotype, we did not find a correlation between the density of NK cells either in the invasive margin or in the stroma of tumors and the clinical outcome of patients.Citation6 Immunohistochemistry analysis of our cohort of patients revealed that NK cells are also present in non tumoral lung tissues, where they have a phenotype similar to NK cells from peripheral blood. It is therefore tempting to speculate that NK cells might have been present and have antitumoral cytotoxic activity at very early stages of tumor development and that their phenotype was progressively altered during tumor progression.
It cannot be excluded that intratumoral NK cells may have deleterious effects on other immune cells located in the tumor microenvironment. For instance they might cause inefficient DC maturation because of the lack of IFN-γ secretion by NK cells. A concomitant analysis of immature DC and NK cells in situ would help to reinforce this hypothesis, since we have already observed the presence of immature DC in the stroma of NSCLC.Citation8 NK cells could also favor the recruitment of regulatory T cells, that have been described in the tumor environment,Citation9 through CCL22 chemokine secretion, as it has been observed in a murine model.Citation10
Concluding Remarks
A question of high importance is now to determine if the modifications of intratumoral NK phenotype and functions are reversible. If that is the case, enhancing NK cell function with immunostimulatory cytokines, such as IL-15, IL-18 and type I IFN or by neutralization of immunosuppressive factors produced in the environment might further ameliorate the clinical outcome of NSCLC.
References
- Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Gröne EF, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res 2006; 12:718 - 25; http://dx.doi.org/10.1158/1078-0432.CCR-05-0857; PMID: 16467081
- Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol 2009; 183:4921 - 30; http://dx.doi.org/10.4049/jimmunol.0901226; PMID: 19801517
- Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609 - 22; http://dx.doi.org/10.1172/JCI45816; PMID: 21841316
- Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011; 17:700 - 7; http://dx.doi.org/10.1038/nm.2366; PMID: 21552268
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410 - 7; http://dx.doi.org/10.1200/JCO.2007.15.0284; PMID: 18802153
- Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011; 71:5412 - 22; http://dx.doi.org/10.1158/0008-5472.CAN-10-4179; PMID: 21708957
- Fridman WH, Galon J, Dieu-Nosjean MC, Cremer I, Fisson S, Damotte D, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol 2010; 344:1 - 24; http://dx.doi.org/10.1007/82_2010_46; PMID: 20512556
- Sautès-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I, et al. Tumor microenvironment is multifaceted. Cancer Metastasis Rev 2011; 30:13 - 25; http://dx.doi.org/10.1007/s10555-011-9279-y; PMID: 21271351
- Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol 2010; 5:585 - 90; PMID: 20234320
- Mailloux AW, Young MR. NK-dependent increases in CCL22 secretion selectively recruits regulatory T cells to the tumor microenvironment. J Immunol 2009; 182:2753 - 65; http://dx.doi.org/10.4049/jimmunol.0801124; PMID: 19234170