Abstract
The enzymatic activity of CD73 produces immune-suppressing adenosine. In CD73 deficient hosts, tumor growth and tumor infiltration by Tregs and type 2 immunosuppressive macrophages is reduced. Pharmacological inhibition of CD73 in wild-type mice has similar tumor-suppressing effects. Host CD73 on leukocytes and endothelial cells is thus detrimental for the anti-tumor immunity.
CD73/ecto-5′-nucleotidase is a cell-surface protein expressed on a subset of leukocytes, including CD4+CD25+FoxP3+ Tregs, vascular and lymphatic endothelial cells and certain epithelial cells.Citation1 It is an ecto-enzyme, which dephosphorylates extracellular AMP into adenosine ().Citation2-Citation4 This reaction is an integral part of the adenosinergic signaling pathway that encompasses the following sequential hydrolyzing reactions: ATP → ADP → AMP → adenosine → inosine. ATP and ADP generally give rise to pro-inflammatory signals via purinergic P2X and P2Y receptors. Adenosine, in contrast, binds to adenosine receptors and evokes anti-inflammatory responses. The enzymatic activity of CD73 is involved in the regulation of leukocyte extravasation, vascular barrier function, and immunosuppressive functions of Tregs, which are all relevant to tumor immunity.
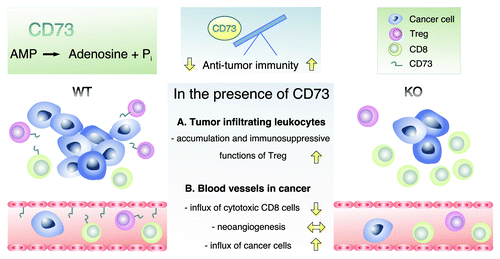
Figure 1. The CD73 on hematopoetic and non-hematopotic cells of the host regulates anti-tumor immunity. The enzymatic activity CD73 is depicted at the top. The involvement of endothelial and leukocyte CD73 in leukocyte extravasation and immune suppression in wild-type and CD73-deficient mice are illustrated. CD73 regulates recruitment of both CD73-positive and -negative leukocytes by modulating the endothelial adhesion molecules and permeability, and CD73 may also have direct adhesive functions. Immune suppression is mainly mediated through the production of adenosine. In addition, certain cancer types express CD73, and it augments the migration of these malignant cells and further renders the tumor microenvironment more immune-suppressing.
CD73 can also be expressed on certain cancer cell types.Citation5,Citation6 These include leukemia, glioblastoma, melanoma, ovarian, gastric, colon and breast cancer. In these cells CD73 activity confers increased migratory and invasive capacity and augments neovascularization of the tumors. Inhibition of cancer cell CD73 activity can impair tumor progression.
The potential role of host CD73 in tumor growth has not been addressed. In our study, we used CD73-negative tumor cells (B16 melanomas) and CD73-deficient mice to dissect the contribution of host CD73 to the tumor progression and anti-tumor immunity.Citation7
Although the majority of both CD4+ and CD8+ T-cells in the lymph nodes normally express CD73, the absence of CD73 did not alter their numbers.Citation7 Nevertheless, the extracellular adenosinergic signaling cascade of T-lymphocytes was abnormal in CD73-deficient mice. CD73-negative T-cells showed much higher ATPase and ADPase activities, and practically no ecto-5′-nucleotidase activity when compared with the wild-type controls. Thus, since the ATP and ADP hydrolyzing activities are increased, and the dephosphorylation of AMP into adenosine is reduced in the CD73-deficient mice, the net effect appears to be the accumulation of AMP.
The growth of primary subcutaneous tumors from CD73-negative melanoma cells, and their metastases to the draining lymph nodes were attenuated in CD73-deficient hosts.Citation7 Since CD73 is normally expressed both on the endothelium and on leukocytes, we next studied which of these cell types would be relevant for the altered anti-tumor responses.
Since adenosine is proangiogenic, the CD73 deficiency might result in an inefficient angiogenic switch in tumors. We found that CD73 is indeed induced in a subpopulation of neoangiogenic vessels within the melanomas.Citation7 However, there was no difference in the numbers of blood or lymphatic neovessels within the tumors when comparing CD73-deficient and wild-type mice. Nevertheless, binding of isolated wild-type tumor-infiltrating leukocytes to CD73-deficient tumor vasculature was impaired by ~50% when compared with the binding to the wild-type CD73 expressing tumor vasculature in in vitro adhesion assays. Thus, CD73 appears not to be necessary for the formation of tumor neovessels, but is expressed on those, and may contribute to the leukocyte immigration into the tumors ().
The lack of CD73 might also alter immune-suppressing functions of tumor infiltrating leukocytes. To study this alternative, we enumerated different leukocyte subtypes from the tumors. We did not observe any genotype-specific differences in the overall numbers of intratumoral CD4+ T-helper cells, CD8+ T-cytotoxic cells or F4/80+ macrophages.Citation7 However, the tumors grown in CD73-deficient hosts had significantly fewer FoxP3+ lymphocytes and macrophage mannose receptor-positive (type 2) macrophages in the tumors. Moreover, in microarray analyses the tumor infiltrating leukocytes in the CD73-deficient mice had more IFNγ and NOS2 mRNA (both markers of type 1 polarized macrophages) than the wild-type controls. Together these data suggest that the intratumoral accumulation of immune suppressive cell types, Tregs and type 2 macrophages, is compromised in the absence of host CD73 ().
Adenosinergic signaling can be manipulated pharmacologically in vivo. Apyrase hydrolyzes ATP and ADP into AMP, and AMPCP, a non-hydrolyzable nucleotide analog, inhibits CD73 activity. We observed that peritumoral injections of either apyrase or AMPCP significantly retarded melanoma progression and inhibited accumulation of immune-suppressing cell types in wild-type mice.Citation7 The same drugs had no effect on tumor growth in CD73-deficient hosts. Thus, pharmacological lowering of peritumoral ATP levels or adenosine production phenotypically reproduced the effects seen in gene deletion experiments.
Recently, these findings have been recapitulated by two independent groups.Citation8,Citation9 They show that ablation of host CD73 suppresses the growth of several tumor cells types in vivo. At least in OVA-expressing model tumors, lack of host CD73 results in increased accumulation of antigen-specific, IFNγ producing CD8+ T cells in the tumors. In Tregs CD73 was shown to be functionally important for their mune-suppressing functions in tumors. Moreover, the endothelial CD73 was involved in the recruitment of both anti-tumor leukocytes and blood-borne metastatic melanoma cells also in vivo.
Thus, the emerging concept is that CD73 on both host and tumor cell side is involved in tumor progression (). Tumor cell CD73 enhances the migratory properties of malignant cells and contributes to the immuno-evasion. On the host leukocytes, CD73 activity also contributes to the immunosuppression. On normal and neoangiogenic vessels, CD73 regulates the influx of leukocytes. Inhibition of CD73, which is induced under hypoxic conditions in tumors, by enzyme inhibitors, siRNA or function blocking monoclonal antibodies results in attenuated tumor progression. Therefore, ectoenzymatic modulation of cancer may offer attractive therapeutic options in future.
References
- Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2006; 2:351 - 60; http://dx.doi.org/10.1007/s11302-005-5302-5; PMID: 18404475
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 2008; 1783:673 - 94; http://dx.doi.org/10.1016/j.bbamcr.2008.01.024; PMID: 18302942
- Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol 2009; 30:102 - 8; http://dx.doi.org/10.1016/j.it.2008.12.002; PMID: 19201652
- Jalkanen S, Salmi M. VAP-1 and CD73, endothelial cell surface enzymes in leukocyte extravasation. Arterioscler Thromb Vasc Biol 2007; 28:18 - 26; http://dx.doi.org/10.1161/ATVBAHA.107.153130; PMID: 17962625
- Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 2010; 29:5346 - 58; http://dx.doi.org/10.1038/onc.2010.292; PMID: 20661219
- Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res 2010; 70:6407 - 11; http://dx.doi.org/10.1158/0008-5472.CAN-10-1544; PMID: 20682793
- Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemela J, Laurila JP, Elima K, et al. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol 2011; 41:1231 - 41; http://dx.doi.org/10.1002/eji.201041292; PMID: 21469131
- Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest 2011; 121:2371 - 82; http://dx.doi.org/10.1172/JCI45559; PMID: 21537079
- Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 2011; 71:2892 - 900; http://dx.doi.org/10.1158/0008-5472.CAN-10-4246; PMID: 21292811