Abstract
Innate immunity serves as a first line of defense against infectious agents, and germ-line-encoded pattern recognition receptors detect stressed and infected cells and elicit potent effector activities that accomplish efficient microbe containment. Recent evidence demonstrates that these pattern-sensing systems are also applicable to the recognition of tumor-derived stress-related factors. In particular, toll-like receptors and cytosolic sensors for DNA and RNA recognition utilize endogenous host elements containing microbial components, danger-associated molecules, and/or nucleic acids to stimulate innate signaling pathways and generate protective immune responses against nascent tumors in animal models and humans. In this review, we describe recent advances and perspectives about antitumor mechanisms and clinical application of innate immune signals and pathways.
Introduction
Innate immunity serves as a first line of defense against infection, as germ-line encoded pattern recognition receptors (PRRs) rapidly detect stressed or infected cells, thereby triggering potent effector mechanisms aimed at accomplishing efficient microbe containment.Citation1 Although the importance of innate immune signals in sensing microbes has been established, the molecular machineries whereby innate immunity communicates with oncogenic stresses and regulates tumorigenesis remain elusive. In this review, we oversee the role of each PRRs and their effectors in generating protective antitumor immunity and their potential clinical application.
The Role of TLRs in Antitumor Immunity
Toll-like receptors (TLRs) serve as pattern recognition receptors that recognize conserved structures of pathogens and endogenous compounds known as “danger signals.”Citation2 Since the initial observation by William Coley that dying bacterial components display antitumor capacities, it has been becoming apparent that TLRs function as essential and minimal elements in boosting antitumor immunity as adjutants by utilizing various sets of microbial components and endogenous host elements. Most notably, TLR stimulation breaks the tolerogenic status of antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs), to tumor self-antigens, through the upregulation of costimulatory molecules and proinflammatory cytokines, and triggers innate and adaptive arms of effector responses.Citation3,Citation4 In particular, type I interferons (IFNα/β), upon the recognition of subsets of TLR (TLR3, TLR4, TLR7, TLR8, TLR9), has been fully defined as their tumor suppressive effectors.Citation5,Citation6 Mice with targeted mutations of the type I interferon receptors or wild type animals administered neutralizing antibodies to type I IFN manifested enhanced susceptibility to chemical carcinogenesis and tumor transplantation.Citation7 Protection in these systems involved host immunity and p53 tumor suppressor function in cancer cells.Citation8 Thus, IFNα/β mediates critical though distinct functions in tumor immune surveillance.
Furthermore, recent studies have explored that TLR ligands in antigen-presenting cells preferentially target ingested apoptotic cells to endosomal pathways in dendritic cells, and support forming peptide-MHC class II complex and enhance the recognition of immunogenic targets by antigen-specific T lymphocytes.Citation9,Citation10 In this regard, the administration of TLR agonists may sense APCs to facilitate cross-presentation of immunogenic tumor antigens and trigger specific T cell responses ().
Figure 1. TLR and DC cross-presentation of tumor antigens. Upon pahgocytosis of dying tumor cells, the cargo is internalized into phagosomes. If microbial or endogenous danger signals serving as TLR agonists are contained in tumor cells, the engagements of TLR along with phagosomal membranes facilitates the assembly of phagosomal-lysosomal fusion machinery and the processing of tumor-associated antigens, and makes MHC class II amenable to binding of antigenic peptides. The resultant peptide-MHC comlex enables tumor-specific CD4+T cells to recognize and kill tumors.
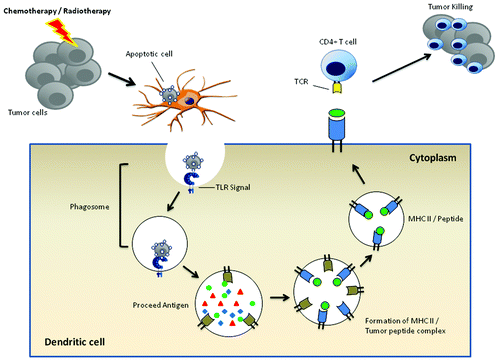
Consistent with the positive role of TLR in delineating protective antitumor immunity, the possibility of TLR ligands as anticancer therapies has been under intense investigation in preclinical studies. The stimulation of TLR8 with its ligands (Poly-G3 and ssRNA compounds) markedly attenuated suppressive activities of regulatory Foxp3+T cells, which serve as key players in restraining effective antitumor immune responses.Citation11,Citation12 Therefore, the manipulation of TLR8 signaling and functions may be suitable therapeutic strategies for restraining Treg cell functions and improving the efficacy of cancer immunotherapy.
The application of synthetic TLR7 agonists, imiquimod, effectively eradicates superficial basal cell carcinomas.Citation13 TLR7 also serves as a receptor for certain sets of siRNA to stimulate IFNα secretion in pDC, enabling simultaneous target of oncogene and innate immunity.Citation14
As mechanisms of action, TLR8 and TLR7 similarly causes secretion of IFN-α and other proinflammatory cytokines through activation of MyD88-TRAF6 mediated pathways in plasmacytoid dendritic cells (pDC), and stimulate various components of innate and adaptive immune systems.Citation12 Moreover, TLR-7/8 agonists also render dendritic cells to acquire cytotoxic activities against tumor cells in TRAL-dependent mechanisms.Citation15
CpG oligonucleotide (ODN) that target TLR9 signals are also evaluating as a suitable candidate to stimulate antitumor immune responses. CpG ODN is composed of three subclasses that have different structural and biological properties: In particular, A-class CpG ODN triggers massive secretion of IFNα in pDC, and B-class ODN stimulates B cells but induce relatively little pDC secretion of IFNα. Any class of CpG oligonucleotide stimulates immune cells that constitutively express TLR9, as B cells and pDCs, with a predominant pattern of Th1 cytokine and chemokines secretion, serving TLR9 agonists as strong Th1 vaccine adjuvant.Citation16 However, recent studies have revealed that A-class ODN mainly targets pDC to secrete IFNα and activate NK cells, while simultaneously mediate immune suppressive effects through induction of indolamine 2,3-dioxygenase (IDO)Citation17,Citation18 and generation of Tregs.Citation19 On the other hands, B-class ODN sensitizes naive B cells to antigenic stimuli and promotes the differentiation them into antibody-producing plasma cells, driving strong Th1 T cell responses.
Several combinatorial strategies of CpG ODN with peptide vaccines, cell vaccines, chemotherapy, showed some clinical efficacy in reducing tumor burden and prolonging patient’s survival.Citation20,Citation21 These encouraging results in clinical trials with CpG ODN provides a rationale of using TLR agonists to accelerate antitumor reactions in patients, although it remains unclear whether they are sufficient to overcome multiple layers of immune evasion systems accumulated at tumor microenvironments.
TLR4 agonists also create pro-inflammatory milieu at tolerogenic tumor environments, through production of various cytokines and chemokines. The therapeutic efficacy of adoptive antitumor immunotherapy relied on the activation of enteric microbiota and subsequent activation of TLR4 signals.Citation22 Furthermore, TLR4 signals trigger immunogenicity of apoptotic tumor cells induced by chemotherapeutic agents, due to interaction with HMGB-1. Cancer patients who carried a TLR4 loss-of-function allele relapsed much quicker following chemotherapy compared with those who have wild type copies, demonstrating that TLR4 agonists potentiate immunogenicity and clinical efficacies of anticancer therapies in certain subsets of patients.Citation23,Citation24
TLR3 agonists activate IFN and NFκB signals, and stimulate antitumor immune responses. Myeloid dendritic cells (mDC) stimulate IFNγ secretion and cytotoxicity of NK cells through coordinated interplay of TLR3 and MDA5-dependent dsRNA recognition.Citation25,Citation26 The TLR3 agonists showed an excellent antitumor efficacy in preclinical models.Citation27,Citation28
Taken together, these findings highlight the therapeutic potential of TLR agonists as new types of adjuvant that strongly stimulate durable antitumor immune responses.
The Role of RLHs in Antitumor Immunity
Recent studies have been validating the utility of targeting cytosolic PRRs, RIG-I like helicase pathways (RLHs), in generating antitumor responses. RLHs trigger the production of IFNα/β, the critical antitumor innate cytokines, but also coordinate tumor cell apoptosis in cell-autonomous fashion. Indeed, the introduction of RIG-I or MDA5 ligands mimetic into human melanoma cells can stimulate the mitochondrial pathway of apoptosis in BH3-only protein Noxa-dependent but IFNα-independent way, culminating for tumor cell death in the cell-autonomous manner.Citation29 In contrast, the targeted delivery of 3p-RNA enables ovarian tumor cells to activate IFN-mediated innate signals and induce various immunogenic cytokines and chemokines, leading to apoptotic tumor cell death and ingestion by APC.Citation30 These findings demonstrate that RIG-I may delineate immunologic or non-immunologic pathways in triggering intrinsic tumoricidal responses in coordinate and distinct way ().
Figure 2. Intrinsic and immune-mediated antitumor actions of RIG-I. The RIG-I-mediated triggering of cytosolic PRRs results in the coordinated activation of IFNα/β-dependent innate responses and mitochondrial death pathways mainly mediated by Bcl-x-related protein Puma and Noxa, triggering tumor killing through the cell-autonomous and immune-mediated mechanisms.
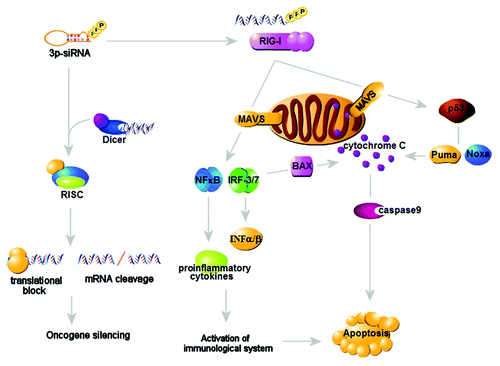
Other studies explored the possibility of RIG-I-mediated recognition of innate immunity in boosting antitumor responses. The synthetic Bcl-2-targeted siRNA conjugated to RIG-I ligand 3pRNA results in the profound antitumor responses through inhibition of Bcl-2 and RIG-I-dependent IFNα secretion in tumor cells.Citation31,Citation32 Furthermore, RIG-I/MDA-5 pathways are involved in antitumor effect of synthetic retinoic acid CD437.Citation33
Taken together, cytosolic PRRs may serve as therapeutic targets to activate innate responses, and possibly overcome immunosuppressive environments arising in established tumors.
The Role of NLRs in Antitumor Immunity
NOD-like receptor (NLR) family proteins are characterized by their broad sensing systems to recognize not only PRRs but also endogenous host molecules associated with inflammation termed as danger-associated molecular pattern (DAMP), including uric acids, alum salt, silica.Citation34,Citation35 In this context, NLRs may serve as host defense systems involved in infectious and sterile inflammation caused by pathogens and environmental insults, respectively. Indeed, the mutation of NLR gamily protein NOD2 is associated with susceptibility of inflammatory bowel disease (IBD), which is manifested by chronic colonic inflammation and deregulated host response to enteric commensal microbiota and endogenous stresses.Citation36,Citation37 However, although IBD frequently cause cancer development in the background of unresolved inflammations, whether NOD2 is involved in tumorigenesis remains obscure. In this regards, the molecular mechanisms by which the interplay between NOD2 sensing systems and host microenvironments regulate carcinogenesis is required for further clarification.
On the other hands, recent studies unveiled the protective functions of NOD1 in tumorigenesis. The disruption of NOD1 functions rendered MCF-7 breast cancer cells to resist apoptotic cell death and promote in vivo tumor growth.Citation38 Moreover, NOD1 deficiency caused the progression of colon carcinogenesis in the background of carcinogen exposure or Apc mutation. As mechanisms of tumor-protective effect, NOD1 signaling pathway positively regulates intestinal tissue integrity as well as restrains deregulation of commensal microbiota followed by disrupted barrier function.Citation39 These findings further highlight the importance of NOD1 proteins in linking host innate immunity with tissue homeostasis.
Other NLR family proteins, called inflammasomes, that regulate the processing and maturation of IL-1β and IL-18, is critical to regulate proper innate immune responses to various environmental insults, including gout, type2 diabetes.Citation40-Citation42 Recent reports demonstrated that the inflammasome protein NLRP3 serves as a negative regulator of tumorigenesis during carcinogen-induced colon cancer.Citation43 In this study, NLRP3 protects colonic tissues upon chemical insults in caspase-1 and IL-1β-dependent fashion.
Besides the role of NLRP3 in regulating inflammation and colon cancer, inflammasomes may be associated with broad modes of carcinogenesis and antitumor immunity. Recent report implicates that NLRP agonist ATP plays a role in recruiting phagocytes to facilitate phagocytic removal of apoptotic cells.Citation44 In addition, ATP further boosts innate responses in coordination with PPRs by interacting with thioredoxin-interacting protein TXNIP and generating ROS.Citation45 In this regard, it is possible that upon the recognition of dying tumor cells with phagocytosis, the release of ATP from dying tumor cells senses DCs to activate NLRP3 inflammasome and secrete IL-1β, by which antitumor effector CD8+ T cells were efficiently primed to induce antitumor immunity (). Indeed, recent study validated the role of ATP from chemotherapy-sensitized tumor cells in stimulating NLRP3-mediated IL-1β and tumor-specific CD8+T cell responses.Citation46 In addition, the oncogenic pathways, such as Bcl-2 anti-apoptotic families, as well as autophagic responses, may influence the biologic activities of inflammasome pathways by interacting with key signaling components,Citation47,Citation48 underscoring the broad and heterogeneous functions of inflammasomes in regulating various modes of stress responses in tumor cells.
Figure 3. NLRP3-mediated antitumor responses. ATP and PRRs released from dying tumor cells consequent to cytotoxic therapies send the “find-me” signals to dendritic cells to facilitate the uptake of apoptotic tumor cells, and trigger the ROS production to activate NLRP3-mediated inflammasome pathways. The resultant activation of inflammasome results in the release of IL-1β, which may contribute to the tumor-specific CTL responses.
AIM2 has been identified as NLR-independent regulators of inflammasome that senses cytoplasmic DNA.Citation49-Citation52 Interestingly, prior to identification as a DNA sensor, AIM2 has been recognized as a tumor suppressor triggering apoptosis and suppressing tumor growth in melanomas.Citation53 As one of the mechanisms by which AIM2 suppress tumorigenicity, AIM2 triggers programmed cell death associated with caspase-1-dependent inflammation, termed as pyroptosis, in tumor cells.Citation52,Citation54 AIM2 also suppresses proliferation of HCT116 colon cancer cells by cell cycle arrest, but induces genes related to tumor invasion and metastasis.Citation55 Taken together, it remains unresolved how AIM2 regulates oncogenesis and antitumor innate responses during the course of tumorigenic process.
Conclusion
In this review, we provide the overview in the role of innate immune signals to regulate protective antitumor responses. Although pattern recognition of microbes is critical to sense various types of cells to orchestrate inflammatory and antimicrobial cascades and to efficiently trigger protective immunity, it remains to be determined whether inflammatory responses consequent to the recognition of microbe and endogenous molecules in tumor cells could be protective or supportive to tumorigenicity. Accumulating evidences have been revealed that the quality of tumor microenvironments may determine the direction of interplay of tumors and host innate immunity throughout the different stages of carcinogenesis.Citation56,Citation57 In this regard, the detailed evaluation of molecular intersection between innate signals and oncogenic pathways should provide the useful insight into the mechanisms of tumor recognition of innate immunity as well as exploration of new therapeutic approaches targeting innate immune signals in future.
Acknowledgments
I thank Dr Shigeki Chiba and Muhammad Baghdadi (Hokkaido University) for generating Figures. This study is partially supported by a Grant-in-Aid for Scientific Research for Young Scientists (A), Scientific Research (C), Innovative Research from Ministry of Education, Culture, Sports Science and Technology (MEXT), Japan.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140:805 - 20; http://dx.doi.org/10.1016/j.cell.2010.01.022; PMID: 20303872
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11:373 - 84; http://dx.doi.org/10.1038/ni.1863; PMID: 20404851
- Watts C, West MA, Zaru R. TLR signaling regulated antigen presentation in dendritic cells. Curr Opin Immunol 2010; 22:124 - 30; http://dx.doi.org/10.1016/j.coi.2009.12.005; PMID: 20083398
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010; 327:291 - 5; http://dx.doi.org/10.1126/science.1183021; PMID: 20075244
- Takaoka A, Tamura T, Taniguchi T. Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci 2008; 99:467 - 78; http://dx.doi.org/10.1111/j.1349-7006.2007.00720.x; PMID: 18190617
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21:137 - 48; http://dx.doi.org/10.1016/j.immuni.2004.07.017; PMID: 15308095
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol 2005; 6:722 - 9; http://dx.doi.org/10.1038/ni1213; PMID: 15951814
- Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, et al. Integration of interferon-alpha/beta signaling to p53 responses in tumor suppression and antiviral defense. Nature 2003; 424:516 - 23; http://dx.doi.org/10.1038/nature01850
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006; 440:808 - 12; http://dx.doi.org/10.1038/nature04596; PMID: 16489357
- Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol 2006; 7:1029 - 35; http://dx.doi.org/10.1038/ni1006-1029; PMID: 16985500
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+regulatory T cell function. Science 2005; 309:1380 - 84; PMID: 16123302
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007; 27:334 - 48; http://dx.doi.org/10.1016/j.immuni.2007.05.020; PMID: 17656116
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7-MyD88-dependent signaling pathway. Nat Immunol 2002; 3:196 - 200; http://dx.doi.org/10.1038/ni758
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med 2005; 11:263 - 70; http://dx.doi.org/10.1038/nm1191; PMID: 15723075
- Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med 2007; 204:1441 - 51; http://dx.doi.org/10.1084/jem.20070021; PMID: 17535975
- Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest 2007; 117:1184 - 94; http://dx.doi.org/10.1172/JCI31414; PMID: 17476348
- Wingender G, Garbi N, Schumak B, Jungerkes F, Endl E, von Bubnoff D, et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol 2006; 36:12 - 20; http://dx.doi.org/10.1002/eji.200535602; PMID: 16323249
- Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. CpG oligonucleotides induce splenic CD19+dendritic cells to acquire potent idolamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol 2006; 175:5601 - 5
- Moseman EA, Liang X, Dawson AJ, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, et al. Human plasmacytoid dendritic cells activated by CpG oligonucleotides induce the generation of CD4+CD25+regulatory T cells. J Immunol 2004; 173:4433 - 42; PMID: 15383574
- Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, Moser A, et al. Phase II trial of toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol 2006; 24:5716 - 24; http://dx.doi.org/10.1200/JCO.2006.07.9129; PMID: 17179105
- Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G, et al. Radomized phase II trial of a toll-like receptor 9 agonist oligonucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small cell lung cancer. J Clin Oncol 2008; 26:3979 - 86; http://dx.doi.org/10.1200/JCO.2007.12.5807; PMID: 18711188
- Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest 2007; 117:2197 - 204; http://dx.doi.org/10.1172/JCI32205; PMID: 17657310
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050 - 9; http://dx.doi.org/10.1038/nm1622; PMID: 17704786
- Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success?. J Clin Invest 2008; 118:1991 - 2001; http://dx.doi.org/10.1172/JCI35180; PMID: 18523649
- Miyake T, Kumagai Y, Kato H, Guo Z, Matsushita K, Satoh T, et al. Poly I:C-induced activation of Nk cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol 2009; 183:2522 - 8; http://dx.doi.org/10.4049/jimmunol.0901500; PMID: 19635904
- McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, et al. Distinct and complementary functions of MDA5 and TLR3 in poly (I:C)-mediated activation of NK cells. J Exp Med 2009; 206:2967 - 76; http://dx.doi.org/10.1084/jem.20091181; PMID: 19995959
- Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, et al. Antitumor NK activation induced by the toll-like receptor 3-TICAM (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci USA 2007; 104:252 - 7; http://dx.doi.org/10.1073/pnas.0605978104; PMID: 17190817
- Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol 2006; 176:4894 - 901; PMID: 16585585
- Besch R, Poeck H, Hohenauer T, Hohenauer T, Senft D, Hacker G, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 2009; 119:2399 - 411; PMID: 19620789
- Kübler K, Gehrke N, Riemann S, Bohnert V, Zillinger T, Hartmann E, et al. Targeted activation of RNA helicase retinoic acid-inducible gene-I induces proimmunogenic apoptosis of human ovarian cancer cells. Cancer Res 2010; 70:5293 - 304; http://dx.doi.org/10.1158/0008-5472.CAN-10-0825; PMID: 20551064
- Poeck H, Besch R, Maihoefer C, Maihoefer C, Renn M, Tormo D, et al. 5′-Triphosphate-siRNA turning gene silencing and Rig-I activation against melanoma. Nat Med 2008; 14:1256 - 63; http://dx.doi.org/10.1038/nm.1887; PMID: 18978796
- Anz D, Koelzer VH, Moder S, Thaler R, Schwerd T, Lahl K, et al. Immunostimulatory RNA blocks suppression by regulatory T cells. J Immunol 2010; 184:939 - 46; http://dx.doi.org/10.4049/jimmunol.0901245; PMID: 19966212
- Pan M, Geng S, Xiao S, Ren J, Liu Y, Li X, et al. Apoptosis induced by synthetic retinoic acid CD437 on human melanoma A375 cells involves RIG-I pathway. Arch Dermatol Res 2009; 301:15 - 20; http://dx.doi.org/10.1007/s00403-008-0902-x; PMID: 18936944
- Ting JP, Duncan JA, Lei Y. How the non-inflammasome NLRs function in the innate immune system?. Science 2010; 327:286 - 90; http://dx.doi.org/10.1126/science.1184004; PMID: 20075243
- Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol 2009; 4:365 - 98; http://dx.doi.org/10.1146/annurev.pathol.4.110807.092239; PMID: 18928408
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001; 411:603 - 6; http://dx.doi.org/10.1038/35079114; PMID: 11385577
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezaed JP, Belaiche J, et al. Association of NOD2 leucin-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001; 411:599 - 603; http://dx.doi.org/10.1038/35079107; PMID: 11385576
- da Silva Correia J, Miranda YY, Austin-Brown N, Hsu J, Mathison J, Xiang R, et al. Nod1-dependent control of tumor growth. Proc Natl Acad Sci USA 2006; 103:1840 - 5; http://dx.doi.org/10.1073/pnas.0509228103; PMID: 16446438
- Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res 2008; 68:10060 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-08-2061; PMID: 19074871
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237 - 41; http://dx.doi.org/10.1038/nature04516; PMID: 16407889
- Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger?. Science 2010; 327:296 - 300; http://dx.doi.org/10.1126/science.1184003; PMID: 20075245
- Schroder K, Tshopp J. The inflammasome. Cell 2010; 140:821 - 32; http://dx.doi.org/10.1016/j.cell.2010.01.040; PMID: 20303873
- Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 2010; 207:1045 - 56; http://dx.doi.org/10.1084/jem.20100050; PMID: 20385749
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SG, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009; 461:282 - 6; http://dx.doi.org/10.1038/nature08296; PMID: 19741708
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010; 11:136 - 40; http://dx.doi.org/10.1038/ni.1831; PMID: 20023662
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170 - 8; http://dx.doi.org/10.1038/nm.2028; PMID: 19767732
- Faustin B, Chen Y, Zhai D, Negrate GL, Lartigue L, Satterthwait A, et al. Mechanisms of Bcl-2 and Bcl-X(L) inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci USA 2009; 106:3935 - 40; http://dx.doi.org/10.1073/pnas.0809414106
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, et al. Loss of Autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008; 456:264 - 8; http://dx.doi.org/10.1038/nature07383; PMID: 18849965
- Bürckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 2009; 10:266 - 72; http://dx.doi.org/10.1038/ni.1702; PMID: 19158679
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009; 458:509 - 13; http://dx.doi.org/10.1038/nature07710; PMID: 19158676
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009; 458:514 - 8; http://dx.doi.org/10.1038/nature07725; PMID: 19158675
- Roberts TL, Idris A, Kelly JA, Burnton CM, Hodgson S, Hardy LL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 2009; 323:1057 - 60; http://dx.doi.org/10.1126/science.1169841; PMID: 19119196
- DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 1997; 15:453 - 7; http://dx.doi.org/10.1038/sj.onc.1201206; PMID: 9242382
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009; 7:99 - 109; http://dx.doi.org/10.1038/nrmicro2070; PMID: 19148178
- Patsps G, Germann A, Gebert J, Dihlmann S. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer 2009; 126:1838 - 49
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860 - 7; http://dx.doi.org/10.1038/nature01322; PMID: 12490959
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883 - 99; http://dx.doi.org/10.1016/j.cell.2010.01.025; PMID: 20303878