Abstract
The CD1d-dependent presentation of lipid antigens to natural killer T (NKT) cells is an integral part of the innate immune system. However, the development of anticancer therapies based on NKT-cell agonists has had limited success so far. Humanizing mice with respect to the CD1d/NKT antigen presentation system will provide a tool to identify novel lipids that exert antineoplastic functions by targeting NKT cells before the initiation of costly and lengthy clinical trials.
Immunostimulatory interventions represent one of a limited number of options for the treatment of incurable tumors and metastases after the failure of conventional surgical, chemotherapeutic, and radiological procedures. CD1d-restricted natural killer T (NKT) cells are an unconventional subset of T cells that co-express a T-cell receptor (TCR) and typical natural killer (NK)-cell receptors. The main population of NKT cells in mammals express identical or near-to-identical TCRs, grating them the appellation of invariant NKT (iNKT) cells.Citation1 As part of the innate immune system, iNKT cells rapidly produce TH1 and TH2 cytokines upon activation, and play critical immunomodulatory functions.Citation1 Initial evidence in support of the antitumor functions of iNKT cells came from a study in which α-galactosylceramide (α-GalCer), a synthetic lipid with robust antineoplastic activity, was shown to operate as a potent activation stimulus for iNKT cells.Citation2 Since then, numerous studies have shown protective roles of iNKT cells against tumors.Citation1,Citation3
Based on promising in vitro and in vivo preclinical results, several clinical trials have been launched to test the antineoplastic potential of α-GalCer. However, the therapeutic success of this approach has been limited,Citation3 most likely due to the divergence between the human and murine immune systems, particularly relative to the CD1d-mediated lipid presentation pathway. As an alternative, the limited efficacy of α-GalCer in humans may be (at least partially) due to the mutual inhibition of TH1 and TH2 cytokines secreted by iNKT cells.Citation1,Citation4,Citation5 Thus, numerous lipids that induced biased TH1 cytokine responses have been tested for their antineoplastic functions in conventional murine tumor models.Citation4 However, it is unknown whether these results can be translated into clinical settings in view of several, apparently subtle but critical, differences between the human and murine CD1d/NKT lipid presentation system.
The first of these differences involves the affinity of CD1d for lipid ligands, in turn affecting the kinetics through which these molecules are loaded onto CD1d.Citation5 Second, the affinity of the CD1d/lipid complex for the TCR of iNKT cells is substantially different between in human and mouse systems. The affinity of human CD1d complexed with α-GalCer for cognate iNKT TCR is indeed approximately 50-fold lower than that its murine counterpart.Citation6 Third, the F’ lipid-binding groove of human CD1d is significantly longer as compared with that of mouse CD1d. Fourth, unlike murine CD1d, human CD1d harbors a bulky tryptophan residue on top of the lipid-binding groove, resulting in a steric hindrance for the positioning of the galactose head group, which most likely affects the binding of CD1d to α-GalCer.Citation5,Citation7 Moreover, the intracellular trafficking of human and mouse CD1d molecules differs, presumably owing to their different affinity with adaptor protein 3 (AP3).Citation1,Citation8 Finally, the abundance and cell surface phenotype of iNKT cells exhibit substantial variations across these 2 species, in particular relative to CD4 expression levels.Citation1
To identify novel lipids compatible with human CD1d and hence that may efficiently stimulate human NKT cells, we aim to build a mouse model bearing a humanized CD1d/NKT lipid presentation system (). As a first step, we generated a human CD1D knock-in (hCD1d-KI) mouse model.Citation9 hCD1d-KI mice express human CD1d under the control of the endogenous Cd1d promoter, ensuring a native expression pattern. Importantly, the expression of human CD1d in mice supports the development of a NKT cell population that closely resembles the human one in abundance and phenotype. One major flaw of evaluating NKT cell-targeting lipids with conventional mouse models is the relative abundance of murine iNKT cells among all lymphocyte populations, which is approximately 10-fold higher than in humans.Citation1,Citation9 hCD1d-KI mice therefore represent a unique model to evaluate the immune responses to lipid CD1d ligands and the antitumor functions of iNKT cells in a physiologically relevant setting. Moreover, the vast majority of iNKT cells in hCD1d-KI mice express the mouse Vβ8 iNKT TCR, the closest homolog of the human Vβ11 TCR β chain. Furthermore, hCD1d-KI mice exhibit an iNKT-cell population characterized by CD4+/CD4−CD8− cell ratio similar to that of its human counterpart. In humans, CD4−CD8− iNKT cells play distinct effector and immunoregulatory roles as compared with CD4+ iNKT cells.Citation1 Importantly, we demonstrated that α-GalCer mediates potent anti-melanoma functions in hCD1d-KI mice.Citation9 This provides the first evidence that human CD1d can present a potent lipid ligand in vivo, and stimulate effective antitumor functions by iNKT cells at a cell abundancy comparable to that found in most humans.
Figure 1. Humanizing mice for identification of novel drugs targeting human iNKT cells for anticancer therapies. α-galactosylceramide (α-GalCer) can potently stimulate the antitumor activity of invariant natural killer T (iNKT) cells in wild-type mice (left). Due to the high affinity of human CD1d to murine iNKT T-cell receptors (TCRs), α-GalCer exhibits robust antitumor functions also in hCD1d-KI mice (central left). Novel α-GalCer analogs that will demonstrate potent antitumor activity in vivo in models incorporating both human CD1d and human iNKT TCRs (central right) will be most promising candidates for iNKT cell-based anticancer immunotherapy (right).
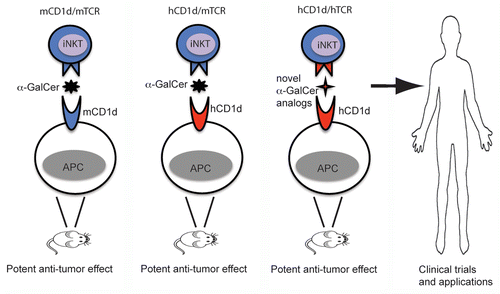
To the best of our knowledge, hCD1d-KI mice currently represent the closest recapitulation of the human CD1d/NKT lipid presentation system in a mouse model. Nevertheless, in hCD1d-KI mice the TCRs expressed by iNKT cells are of murine origin, and the affinity of human CD1d for mouse Vβ8.2 iNKT TCRs is still substantially higher than that of human CD1d for human iNKT TCRs (as determined for clone NKT-15).Citation6 If such a difference in affinity is true for the TCRs of most (if not all) iNKT cell populations in hCD1d-KI mice and humans, it would explain the robust antitumor functions of iNKT cells stimulated with α-GalCer we observed in these miceCitation9 (), contrasting with the low potency of α-GalCer in clinical trials.Citation3 Incorporating TCRs from human iNKT cells into this model is required to more precisely study lipid presentation by the human CD1d/NKT system in vivo (). Transgenic mice expressing a human Vα24Jα18-coding transgene have already been generated.Citation10 Introducing the Vα24Jα18-coding gene into hCD1d-KI mice will allow us to further humanize the CD1d/NKT lipid presentation system of these animals. Lipids that stimulate potent antitumor responses when presented by human CD1d in such an improved hCD1d-KI mouse model have great potential as drug candidates for human iNKT cell-based immunotherapy. Moreover, this model may provide mechanistic details on the factors that determine iNKT-cell responses, hence facilitating the rational design of potent lipid ligands targeting human iNKT cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol 2004; 22:817 - 90; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104608; PMID: 15032598
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997; 278:1626 - 9; http://dx.doi.org/10.1126/science.278.5343.1626; PMID: 9374463
- Exley MA, Nakayama T. NKT-cell-based immunotherapies in clinical trials. Clin Immunol 2011; 140:117 - 8; http://dx.doi.org/10.1016/j.clim.2011.04.015; PMID: 21592864
- Banchet-Cadeddu A, Hénon E, Dauchez M, Renault JH, Monneaux F, Haudrechy A. The stimulating adventure of KRN 7000. Org Biomol Chem 2011; 9:3080 - 104; http://dx.doi.org/10.1039/c0ob00975j; PMID: 21394364
- Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity 2009; 30:888 - 98; http://dx.doi.org/10.1016/j.immuni.2009.03.022; PMID: 19538930
- Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, et al. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity 2009; 31:47 - 59; http://dx.doi.org/10.1016/j.immuni.2009.04.018; PMID: 19592275
- Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, et al. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol 2005; 6:819 - 26; http://dx.doi.org/10.1038/ni1225; PMID: 16007090
- Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, et al. NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. J Exp Med 2003; 197:1623 - 33; http://dx.doi.org/10.1084/jem.20030141; PMID: 12810685
- Wen X, Rao P, Carreño LJ, Kim S, Lawrenczyk A, Porcelli SA, et al. Human CD1d knock-in mouse model demonstrates potent antitumor potential of human CD1d-restricted invariant natural killer T cells. Proc Natl Acad Sci U S A 2013; 110:2963 - 8; http://dx.doi.org/10.1073/pnas.1300200110; PMID: 23382238
- Capone M, Cantarella D, Schümann J, Naidenko OV, Garavaglia C, Beermann F, et al. Human invariant V alpha 24-J alpha Q TCR supports the development of CD1d-dependent NK1.1+ and NK1.1- T cells in transgenic mice. J Immunol 2003; 170:2390 - 8; PMID: 12594262