Abstract
Understanding the spontaneous immune response of cancer patients is critical for the design of efficient anticancer immunotherapies. The power of integrative tumor immunology approaches allowed for a comprehensive view of the immune system evolution in the course of tumor progression and recurrence. We have demonstrated that tumor-infiltrating immune cells are spatiotemporally regulated, a finding that has profound implications for the development of efficient anticancer immunotherapies.
Historical Perspective
The field of cancer immunology has established a strong foundation over the last century and continues to contradict its detractors. In this context, skepticism has decreased after 3 major recent discoveries. First, it has been clearly demonstrated that oncogenesis proceeds in the context of continuous interactions with immunosurveillance, going through an equilibrium, immunoediting, and escape phase, at least in mouse tumor models.Citation1 Second, the immune response of cancer patients has been shown to critically influence their survival. In particular, tumor infiltration by cells of the adaptive immune system has been attributed a prognostic value that is superior to that of classical tumor staging criteria.Citation2,Citation3 We have previously defined these major immunological parameters associated with patient survival as the “immune contexture”Citation3,Citation4, which we defined as the type, functional orientation, density, and location of adaptive immune cells that infiltrate distinct areas of the neoplastic lesion.Citation2-Citation5 A clinical translation of these findings was the establishment of a new scoring system, called “immunoscore” (IS), based on the abundance of 2 distinct lymphocyte populations (CD3+CD45RO+ and CD3+CD8+ or CD8+CD45RO+ cells) at the tumor center (CT) and at its invasive margin (IM).Citation6 Third, several immunotherapies taking advantage of spontaneous adaptive immune responses achieved remarkable successes, hence generating tremendous enthusiasm. These include the adoptive transfer of tumor-specific T cellsCitation7 and the administration of checkpoint blockade inhibitors,Citation8 such as the FDA-approved anti-cytotoxic T lymphocyte-associated protein 4 (CTLA4) monoclonal antibody ipilimumab as well as hitherto experimental monoclonal antibodies targeting programmed cell death 1 (PDCD1, best known as PD-1) or its ligands.
Advocacy for Integrative Cancer Immunology
Neoplastic lesions develop in a very complex microenvironment comprising fibroblasts, endothelial cells, blood vessels, lymph vessels, immune cells, and soluble factors such as cytokines, chemokines, and many metabolic intermediates. Oncogenesis and tumor progression reflect the complex cellular and molecular interactions of neoplastic cells with the immune system. The tumor microenvironment influences the growth of malignant cells as well as their capacity to progress and form metastases. The staggering complexity of multifactorial diseases such as cancer poses significant challenges to the development of stratified or personalized therapies. The integrated analysis of diverse data sets may circumvent these challenges and provide a better understanding of complex systems like the tumor microenvironment. Data integration and biomolecular network reconstruction are powerful approaches that have allowed us to uncover the molecular mechanisms that underpin the progression and recurrence of colorectal carcinoma (CRC). Bioinformatic resources are now emerging to assist these types of analysis. We have developed tools, such as ClueGO and CluePediaCitation9 to improve the biological interpretation of large data sets. We are now approaching a level at which we can capture the dynamics of complex disease processes. Thanks to such as an integrative approach, we have recently presented a comprehensive view on the evolution of the immune system in the course of tumor progression and recurrence,Citation10 showing that intratumoral immune cells are spatiotemporally regulated.
The Immune Landscape in Human Tumors
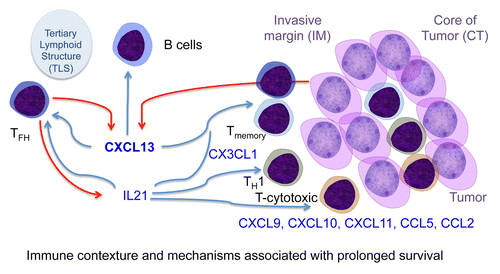
It is of major importance to understand the natural immune response of cancer patients. Combining large-scale approaches, we examined the spatiotemporal dynamics of 28 different types of immune cells that infiltrate human CRCs.Citation10 Our systemic approach to cancer was grounded in the idea that the host immune response and tumor progression reflect perturbations at both the gene and protein level, and that regulatory networks change over time and depending on clinical outcome. To understand the complex spatiotemporal dynamics of the interaction between malignant cells and the immune system in the course of tumor progression, we used several experimental approaches, including immunohistochemical quantification and other visualization methods. We investigated the majority of tumor-infiltrating cells, as well as the sources of genetic diversity, that could influence the generation of immune responses. We built a compendium of mRNAs specific for most innate and adaptive immune cell subpopulations that constituted the “immunome.” We found that the composition of the immune infiltrate, in particular relative to the cells with a major impact on patient survival, changed with tumor stage. The density of follicular helper T (TFH) cells and innate cells increased, whereas that of most other T cell subsets decreased along with tumor progression. B cells, which are key players in the core immune network and associated with prolonged patient survival (at least in this setting), increased at late disease stages, showing a dual effect on disease progression and progression. We demonstrated the positive impact of TFH and B cells against tumor recurrence. In addition, the relevant of the immune system in tumor control was demonstrated in 3 endoscopic-orthotopic colon cancer mouse models. The instability of the gene coding for chemokine (C-X-C motif) ligand 13 (CXCL13) was a mechanism associated with tumor infiltration by TFH and B cells. CXCL13 and interleukin (IL)-21 were indeed pivotal factors for the TFH-B cell axis correlating with patient survival (). Variable densities (“mountains” and “hills”) of immune cell subsets, from the innate and adaptive compartments were illustrated. The tight association of B and T cells could reflect the effect of B cells in modulating T-cell responses, by operating as antigen-presenting cells, providing co-stimulatory signals and secreting cytokines. This integrative study addressed 4 questions. First, which immune cell subpopulations present within the tumor, evolve over time and are associated with tumor progression? Second, which tumor-infiltrating cells most critically influence tumor recurrence and patient survival? Third, which mechanisms are associated with the differential density of intratumoral immune cells? Fourth, can we understand the spatiotemporal dynamics of the immune response, in order to open new therapeutic perspective at different stages of the disease? In conclusion, we revealed the immune landscape of human CRC and the major hallmarks of the microenvironment associated with tumor progression and recurrence. Such a strategy of integrative cancer immunology and the knowledge generated by this study might pave the way to discovering novel efficient immunotherapies.
Figure 1. Immune contexture and mechanisms associated with patient’s prolonged survival. Schematic representation of tumors and major immune cells associated with the proper immune contexture. The tertiary lymphoid structure, TFH and B cell axis, through the production of CXCL13 and IL21 shapes with the chemokines CXCL9, CXCL10, CXCL11, CCL5, and CCL2, the memory, Th1 and cytotoxic T cell response.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Bindea G, Mlecnik B, Angell HK, Galon J. The immune landscape of human tumors: Implications for cancer immunotherapy. OncoImmunology 2013; 2:e27456; 10.4161/onci.27456
References
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331:1565 - 70; http://dx.doi.org/10.1126/science.1203486; PMID: 21436444
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960 - 4; http://dx.doi.org/10.1126/science.1129139; PMID: 17008531
- Galon J, Fridman WH, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res 2007; 67:1883 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-06-4806; PMID: 17332313
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11 - 26; http://dx.doi.org/10.1016/j.immuni.2013.07.008; PMID: 23890060
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298 - 306; http://dx.doi.org/10.1038/nrc3245; PMID: 22419253
- Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232:199 - 209; http://dx.doi.org/10.1002/path.4287; PMID: 24122236
- Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013; 39:49 - 60; http://dx.doi.org/10.1016/j.immuni.2013.07.002; PMID: 23890063
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1 - 10; http://dx.doi.org/10.1016/j.immuni.2013.07.012; PMID: 23890059
- Bindea G, Galon J, Mlecnik B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics 2013; 29:661 - 3; http://dx.doi.org/10.1093/bioinformatics/btt019; PMID: 23325622
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782 - 95; http://dx.doi.org/10.1016/j.immuni.2013.10.003; PMID: 24138885
