Abstract
Upon antigenic stimulation, naïve CD8+ T cells divert their bioenergetic metabolism from oxidative phosphorylation to aerobic glycolysis. This drives CD8+ T cells to differentiate into short-lived effectors, impairing the establishment of immunological memory. The pharmacological inhibition of glycolysis with 2-deoxyglucose enhances the generation of memory CD8+ T cells and thus improves their immunotherapeutic potential against cancer.
The establishment of long-lived CD8+ memory T cells is key for the efficacy of anticancer vaccines and T cell-based immunotherapies.Citation1 It is becoming clear that both the activity and fate of T cells are tightly linked to their metabolic profile, attracting considerable interest around the possibility of targeting metabolism for novel immunotherapeutic applications.
Metabolomic data and real-time bioenergetic analyses have shown that distinct T-cell subsets display unique metabolic programs that are largely determined by their energy demand.Citation2 For instance, quiescent CD8+ T-cell populations such as naïve and memory T cells rely on fatty acid oxidation (FAO) as a primary source for ATP synthesis, as opposed to rapidly proliferating effector T cells that mainly depend on glycolytic metabolism. Whether these metabolic characteristics merely reflect functional changes orchestrated by diverse transcriptional programs or rather instructively dictate T cell fate decisions has just begun to be addressed.
Recent studies have revealed a pivotal role for FAO in the generation and maintenance of memory CD8+ T cells. CD8+ T cells lacking TNF receptor-associated factor 6 (Traf6) display severe defects in FAO and consequently fail to mount a physiological memory response upon infection.Citation3 Conversely, the overexpression of carnitine palmitoyltransferase 1a (Cpt1a), the rate-limiting enzyme of FAO, is sufficient to augment the number of memory CD8+ T cells and hence potentiate recall immune responses.Citation4 These findings clearly demonstrate that modulating FAO can influence the establishment of immunological memory, indicating that changes in metabolism play a direct role in regulating CD8+ T-cell differentiation.
The observation that naïve T cells divert their metabolism from FAO to glycolysis in response to antigenic stimulation was made over 50 y ago, but it still remains unclear why T cells adopt a less efficient pathway for ATP generation under conditions of high energy demand. It has been proposed that the major function of aerobic glycolysis in rapidly proliferating cells is to maintain high levels of glycolytic intermediates to support anabolic reactions.Citation2 However, novel findings indicate that glycolysis is dispensable for T-cell proliferation but rather is required for the post-transcriptional regulation of specific effector function such as interferon γ (IFNγ) production.Citation5 Additionally, recent work has underscored that glycolysis can regulate fate commitment in CD4+ T cells by contributing to the lineage choice between TH17 and regulatory T cells.Citation6 These findings opened the possibility that the level of glycolytic activity might directly influence the ability of activated T cells to become either effector or long-lived memory cells.
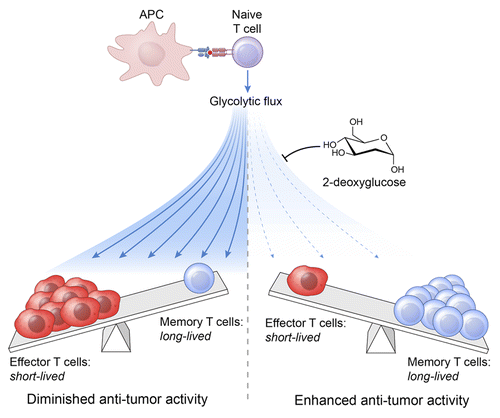
We have recently demonstrated that an increased glycolytic flux drives CD8+ T cells toward a terminally differentiated effector state, while its inhibition preserves the formation of long-lived memory CD8+ T cells ().Citation7 We took three independent approaches to address the role of glucose metabolism in regulating CD8+ T cell fate decisions. First, we used a fluorescent glucose analog, 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]-D-glucose (2-NBDG) to directly measure glucose incorporation in living cells. We found that CD8+ T cells taking up high amounts of glucose exhibit poor engraftment and fail to survive upon adoptive transfer in mice infected with a vaccinia virus expressing the cognate antigen. Conversely, cells exhibiting limited glucose uptake display the molecular signature of memory precursor cells and efficiently establish immunological memory. We next tested whether boosting glycolytic metabolism in T cells would impair their ability to differentiate into memory cells. We found that the overexpression of the glycolytic enzyme phosphoglycerate mutase 1 (Pgam1) boost aerobic glycolysis in CD8+ T cells, resulting in defective memory responses. Lastly, we evaluated whether restraining glycolytic metabolism using the hexokinase inhibitor 2-deoxyglucose (2-DG), would enhance memory T-cell formation. Priming CD8+ T cells in the presence of 2-DG was sufficient to increase the generation of long-lived CD8+ memory T cells and improve recall immune responses. Notably, the direct blockade of glycolysis by 2-DG was associated with an increased expression of transcription factors that support CD8+ T-cell memory, including transcription factor 7 (Tcf7), lymphoid enhancer factor 1 (Lef1), and B cell CLL/lymphoma 6 (Bcl6), as well as an increased activity of forkhead box O1 (Foxo1), a key regulator of memory T-cell differentiation.
Figure 1. Glycolytic metabolism regulates CD8+ T-cell differentiation and effector functions. Upon antigen stimulation, naïve CD8+ T cells undergo massive clonal expansion and differentiate into effector and memory cells. This process is accompanied by metabolic alterations, including an increased flux via aerobic glycolysis. High levels of glycolysis drive CD8+ T cells toward a terminally differentiated effector state that is associated with an impaired antineoplastic activity. Inhibiting glycolysis with 2-deoxyglucose (2-DG) favors the formation of long-lived memory CD8+ T cells that mediate enhanced antitumor responses after adoptive transfer.
Our results are consistent with new findings in CD8+ T cells lacking the von Hippel–Lindau (Vhl) oncosuppressor gene, the main negative regulator of hypoxia-inducible factors (HIFs).Citation8 The elevated activity of HIFs in Vhl-deficient CD8+ T cells stimulate glycolytic metabolism, increase the expression of effector molecules, and hence promote effector functions. Conversely, transcriptional regulators of memory T-cell differentiation including Tcf7 and Bcl6 are downregulated in Vhl−/− CD8+ T cells. Moreover, CD8+ T cells lacking Hif1b displayed increased levels of CD62L and CCR7, 2 lymphoid homing molecules highly expressed in memory T cells, and decreased levels of effector proteins including perforin 1 and granzyme B. This provides additional evidence implicating glycolysis as a pivotal regulator of effector vs. memory T-cell differentiationCitation9. These findings extend beyond mice, as CD8+ T cells from patients bearing gain-of-function mutations in PIK3CD—which encodes p110δ, a phosphoinositide-3-kinase (PI3K) subunit selectively expressed in leukocytes—exhibit enhanced glucose uptake and terminal effector differentiation.Citation10 Altogether, these studies indicate that the glycolytic flux is tightly linked to transcriptional programs regulating effector and memory T-cell differentiation.
It remains largely unknown whether the direct manipulation of the metabolic program of tumor-specific CD8+ T cells can be employed to enhance the efficacy of immunotherapy. Current protocols used to generate T cells for adoptive transfer have the disadvantage of driving cells toward terminal differentiation. We found that blocking glycolysis with 2-DG in the course of ex vivo expansion limits T-cell differentiation, resulting in the generation of antitumor cells with improved fitness. Indeed, T cells primed in the presence of 2-DG accumulated in high amounts within neoplastic lesions and exhibited increased effector functions, as measured in terms of cytokine release and tumor destruction ().Citation7 It is important to note that upon 2-DG withdrawal CD8+ T cells displayed an increased glycolytic program at the tumor site as compared with their vehicle-treated counterparts, indicating that sustained glycolysis is ultimately required for enhanced effector function and antitumor responses. Consistent with our observations, highly glycolytic Vhl-deficient CD8+ T cells display enhanced effector function and are therapeutically superior in experimental models of melanoma.Citation8 Our findings have important implications for the design of both T cell-based immunotherapies and anticancer vaccines, since they provide evidence that targeting cellular metabolism can be an effective strategy to potentiate tumor-targeting immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Sukumar M, Gattinoni L. The short and sweet of T-cell therapy: Restraining glycolysis enhances the formation of immunological memory and antitumor immune responses. OncoImmunology 2013; 2:e27573; 10.4161/onci.27573
References
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 2012; 12:671 - 84; http://dx.doi.org/10.1038/nrc3322; PMID: 22996603
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol 2013; 31:259 - 83; http://dx.doi.org/10.1146/annurev-immunol-032712-095956; PMID: 23298210
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009; 460:103 - 7; http://dx.doi.org/10.1038/nature08097; PMID: 19494812
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012; 36:68 - 78; http://dx.doi.org/10.1016/j.immuni.2011.12.007; PMID: 22206904
- Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013; 153:1239 - 51; http://dx.doi.org/10.1016/j.cell.2013.05.016; PMID: 23746840
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 2011; 208:1367 - 76; http://dx.doi.org/10.1084/jem.20110278; PMID: 21708926
- Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 2013; 123:4479 - 88; http://dx.doi.org/10.1172/JCI69589; PMID: 24091329
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol 2013; 14:1173 - 82; http://dx.doi.org/10.1038/ni.2714; PMID: 24076634
- Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 2012; 209:2441 - 53; http://dx.doi.org/10.1084/jem.20112607; PMID: 23183047
- Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol 2014; 15:88 - 97; http://dx.doi.org/10.1038/ni.2771; PMID: 24165795
