Abstract
Both TH1 and TH2 cytokines influence the antitumor functions of macrophages. We have recently shown that interferon γ (IFNγ) licenses the antineoplastic functions of CD40 ligand (CD40L)-stimulated macrophages more efficiently than interleukin (IL)-4 and IL-13. The presence of a TH1 and TH2 skewed tumor microenvironment may therefore influence therapeutic responses to CD40 agonists, agents that are showing promise in preliminary clinical trials.
The tumor immune microenvironment is dynamic and the abundance and phenotype of tumor-infiltrating immune cells has prognostic significance in patients affected by a number of distinct malignancies.Citation1 In many neoplasms, the immunosuppressive nature of the tumor microenvironment prevents development of a productive anticancer immune response. In line with this notion, the inhibition of specific immunosuppressive pathways within the tumor microenvironment, such as those mediated by programmed cell death 1 (PD-1) and its ligand PD-L1 provide durable clinical benefits to some patients. However, multiple immunosuppressive mechanisms (mediated by various immune cells) operate within the tumor microenvironment and may confer resistance to immunotherapeutic regimens. An alternative approach relies on therapeutic interventions that stimulate tumor-infiltrating immunosuppressive cell populations to adopt a pro-inflammatory phenotype or directly kill malignant cells. Such a repolarization of the tumor microenvironment, which can result in robust anticancer immune responses in in vivo models, may be successfully employed in patients who are relatively insensitive to immune checkpoint inhibitors.
Tumor-associated macrophages (TAMs) constitute a major component of the immune tumor infiltrate. These functionally plastic cells can exist in a spectrum of different phenotypes. M1 and M2 macrophages represent two extremes of such a functional polarization.Citation2 Pro-inflammatory M1 macrophages have direct tumoricidal activity and normally promote antitumor TH1 immune responses. In contrast, M2 macrophages support tumor growth and metastasis, not only by stimulating angiogenesis and the invasive potential of malignant cells, but also by suppressing antitumor TH1 immune responses. The polarization of TAMs is determined by signals from the tumor microenvironment including hypoxia as well as malignant cell- or infiltrating T cell-derived cytokines. While TAMs generally exhibit an M2-like phenotype, the polarization state of macrophages can vary between tumor types as well as in different areas of the same neoplastic lesion.
CD40, a tumor necrosis factor α receptor superfamily member, is widely expressed by cells of the immune system including B cells, dendritic cells (DCs) and macrophages. CD40 agonists inhibit tumor growth in animal models, and promote clinical responses in patients with advanced solid tumors.Citation3 CD40 stimulates antitumor immunity by enhancing the ability of antigen-presenting cells (APCs) to cross-present tumor-associated antigens to CD8+ T cells,Citation4 and by activating TAMs.Citation5,Citation6 The acquisition of tumoricidal activity by TAMs in response to CD40 signaling has been reported to require interferon γ (IFNγ),Citation7 suggesting that a TH1-skewed tumor microenvironment is necessary for CD40-driven antitumor activity. However, human neoplasms are often characterized by a TH2-skewed, rather than TH1-skewed, microenvironment,Citation1 and the influence of TH2 cytokines on human macrophages remains unclear.
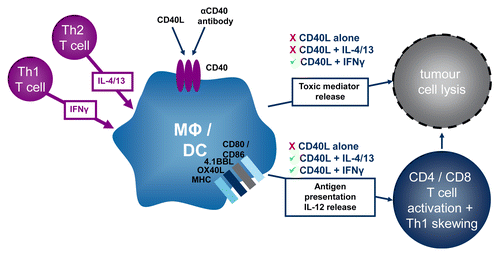
We set out to investigate how TH1 and TH2 cytokines might alter the functional consequences of CD40 activation in macrophages. To this aim, we compared the phenotype of human macrophages differentiated with colony stimulating factor 1 (CSF1, best known as M-CSF) and activated by recombinant CD40 ligand (CD40L) in the presence of either the TH1 cytokine IFNγ or the TH2 cytokines IL-4 and IL-13.Citation8 In the absence of these cytokines, CD40-activated macrophages have a very limited ability to kill tumor cells and produce high levels of the immunosuppressive cytokine IL-10. In line with previous reports,Citation9 we observed that the activation of CD40 in the presence of IFNγ switched macrophages from producing IL-10 to producing the TH1 cytokine IL-12p70. Also, we found that these macrophages are able to skew allogeneic CD4+ T cells toward a TH1 profile and are endowed with an improved ability to kill neoplastic cells. Conversely, CD40 signaling in the presence of IL-4 and IL-13 resulted in a population of activated macrophages with characteristics intermediate between M1 and M2 . These cells produce both IL-12p70 and IL-10 and cause TH1 T-cell skewing in co-culture with allogeneic CD4+ T cells, yet do not display enhanced tumoricidal activity as compared with macrophages stimulated with CD40L in the absence of TH1 or TH2 cytokines.
Our findings demonstrate that the presence of TH1 or TH2 cytokines determine how macrophages respond to CD40 ligation (). This implies that the tumor microenvironment determines the functional outcome of treatment with CD40 agonists. A TH1-dominated tumor microenvironment may optimally license the antitumor functions of CD40-activated macrophages, including their ability to directly kill malignant cells and the acquisition of a pro-inflammatory phenotype. However, the ability of CD40 signaling to repolarize macrophages from an M2-like to an M1-like phenotype even in the presence of IL-4 and IL-13 suggests that CD40 agonists could provide clinical benefits even when the tumor microenvironment is skewed toward a TH2 profile.
Figure 1. Antitumor functions of macrophages activated by CD40 signaling in the presence of either TH1 or TH2 cytokines. CD40 ligand (CD40L) requires the presence of TH1 (i.e., interferon γ, IFNγ) or TH2 (i.e., interleukin(IL)-4, and IL-13) cytokines to elicit macrophage responses in vitro. In the presence of IFNγ, the ligation of CD40 stimulates macrophages to release IL-12p70, to express increased levels of HLA-DR and CD86 on the cell surface, to promote the secretion of TH1 cytokines by allogeneic CD4+ T cells and to direct kill malignant cells. In the presence of IL-4 and IL-13, the ligation of CD40 causes macrophages to release IL-12p70, to express increased levels of CD86 but not HLA-DR on the cell surface, and to promote the secretion of TH1 cytokines by allogeneic CD4+ T cells, but not to exert direct tumoricidal functions. Thus in vitro, CD40L licenses the antitumor functions of primary human monocyte-derived macrophages more efficiently in the presence of IFNγ than in the presence of IL-4 and IL-13.
In rodent tumor models, CD40-activating antibodies have been reported to synergize with T cell-activating immunotherapeutic interventions. For example, in TH2-skewed renal cell carcinomas, the antitumor activity of CD40 combined with IL-2 or IL-15 correlates with the repolarization of TAMs to an M1-like phenotype and the conversion of the tumor microenvironment towards one with a TH1 profile including enhanced CD8+ T-cell infiltration.Citation10 These data indicate that T-cell activation influences the extent to which CD40 agonists are able to license the antitumor functions of TAMs. Thus, our findings highlight the importance of the tumor microenvironment in determining the antitumor activity of CD40 agonists and suggest a possible mechanism for the reported synergy between CD40 agonists and T cell-activating interventions in animal tumor models.
Disclosure of Potential Conflicts of Interest
Nadia Luheshi, Gareth Davies, and James Legg are employees of MedImmune Ltd, a wholly owned subsidiary of AstraZeneca Ltd and are salaried and own stock and/or options in AstraZeneca.
Citation: Luheshi N, Davies G, Legg J. Understanding the influence of the tumor microenvironment on macrophage responses to CD40 agonists. OncoImmunology 2013; 2:e27615; 10.4161/onci.27615
References
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298 - 306; http://dx.doi.org/10.1038/nrc3245; PMID: 22419253
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889 - 96; http://dx.doi.org/10.1038/ni.1937; PMID: 20856220
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013; 19:6286 - 95; http://dx.doi.org/10.1158/1078-0432.CCR-13-1320; PMID: 23983255
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998; 393:480 - 3; http://dx.doi.org/10.1038/31002; PMID: 9624005
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612 - 6; http://dx.doi.org/10.1126/science.1198443; PMID: 21436454
- Rakhmilevich AL, Buhtoiarov IN, Malkovsky M, Sondel PM. CD40 ligation in vivo can induce T cell independent antitumor effects even against immunogenic tumors. Cancer Immunol Immunother 2008; 57:1151 - 60; http://dx.doi.org/10.1007/s00262-007-0447-4; PMID: 18214476
- O’Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF, Teng MW, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med 2012; 209:1869 - 82; http://dx.doi.org/10.1084/jem.20112738; PMID: 22927549
- Luheshi N, Davies G, Poon E, Wiggins K, McCourt M, Legg J. Th1 cytokines are more effective than Th2 cytokines at licensing anti-tumour functions in CD40-activated human macrophages in vitro. Eur J Immunol 2013; http://dx.doi.org/10.1002/eji.201343351; PMID: 24114634
- Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 2011; 187:1157 - 65; http://dx.doi.org/10.4049/jimmunol.1100889; PMID: 21709158
- Weiss JM, Back TC, Scarzello AJ, Subleski JJ, Hall VL, Stauffer JK, Chen X, Micic D, Alderson K, Murphy WJ, et al. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci U S A 2009; 106:19455 - 60; http://dx.doi.org/10.1073/pnas.0909474106; PMID: 19892741
