Abstract
The biology of fracture healing is better understood than ever before, with advancements such as the locking screw leading to more predictable and less eventful osseous healing. However, at times one’s intrinsic biological response, and even concurrent surgical stabilization, is inadequate. In hopes of facilitating osseous union, bone grafts, bone substitutes and orthobiologics are being relied on more than ever before. The osteoinductive, osteoconductive and osteogenic properties of these substrates have been elucidated in the basic science literature and validated in clinical orthopaedic practice. Furthermore, an industry built around these items is more successful and in demand than ever before. This review provides a comprehensive overview of the basic science, clinical utility and economics of bone grafts, bone substitutes and orthobiologics.
Introduction
The human skeleton has a remarkable ability to regenerate itself after injury. This unique restorative capacity, shared perhaps with only the adult human liver, allows bones to heal at shapes, sizes and strengths essentially equal to their pre-injured forms. At its core, orthopaedic fracture care is an attempt to harness this amazing regenerative capacity and let the body do its work.
Unfortunately, conditions for spontaneous bone healing are not always ideal. For thousands of years, man has recognized the importance of immobilization for fracture healing. Yet even with the efficacy of modern internal fixation techniques, infection, poor vascularity, malnutrition and substantial bone or soft tissue loss can impede effective osteosynthesis.
Modern bone grafts, bone substitutes and bioactive factors attempt to facilitate and enhance the healing process when suboptimal conditions exist. Depending on their properties, preparation and application, bone grafts augment natural healing via osteoinductive, osteoconductive and/or osteogenic mechanisms.Citation1
The field of orthopaedic surgery has experienced unprecedented advancements over the last 20 years, with bone graft and orthobiologics playing a significant role. This review will discuss these autologous, allogenic and synthetic substrates with an emphasis on their contribution to modern fracture care and bone loss.
Fracture Nonunion
While the precise definition of fracture nonunion is controversial, the concept of failed union after an adequate period of healing is well recognized to the orthopaedist. Despite this all-too-common complication, no universally accepted definition of nonunion exists in the orthopedic literature.Citation2 The US Food and Drug Administration (US FDA) defines fracture nonunion as a fracture that is at least nine months old in which there have been no signs of healing for three months. Others define nonunion as a fracture in which a minimum of six months has elapsed without any improvement towards union.Citation3,Citation4 Nonunions must be distinguished from delayed unions, or the eventual bony union of a fracture following an atypically long period of healing.
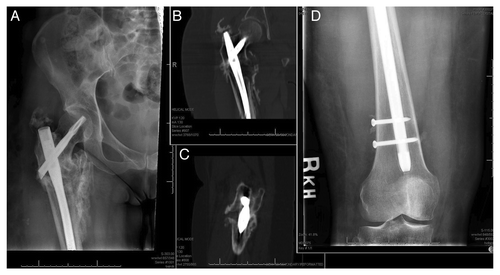
Fracture nonunions are classified based on their radiographic appearance as either hypertrophic or atrophic. These, as well as several other key terms used in this review, are defined in . Hypertrophic nonunions are caused by excessive motion at a fracture site, such as in the setting of insufficient skeletal stabilization. In this situation, the essential biological factors for healing are present; however sustained excess motion at the fracture site prevents bone bridging. An example of hypertrophic nonunion of a subtrochanteric femur fracture can be seen in . Eleven months after injury, excess motion of the cephalomedullary device, secondary to insufficient distal fixation, has prevented bridging of the expansive hard callus. In situations of hypertrophic nonunion, successful healing is achieved by removing the abnormal fibrous, cartilaginous and adipose tissues interposed between the mobile bone ends and by providing a stiffer and sturdier construct across the fracture.1 Additional osteobiologic support for hypertrophic nonunions is typically unnecessary.
Table 1: Definition of terms and common examples
Figure 1: (A) AP Radiograph of a hypertrophic nonunion of a subtrochanteric femur fracture, eleven months following cephalomedullary rod fixation. Coronal (B) and sagittal (C) CT images of the hypertrophic nonunion. (D) AP radiograph of the knee, showing insufficient effectively-unicortical screw fixation, which led to excess implant motion at the fracture site.
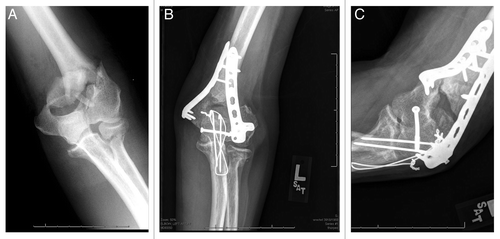
Atrophic nonunions, however, are the result of inadequate biological conditions for healing, and pose a comparatively greater challenge to the orthopaedist. Inadequate vascularity and/or metabolic conditions lead to impaired healing.1 Atrophic nonunions appear on radiographs as blunted bone ends with little to no evidence of osteoid or mineral deposition. An example of an atrophic distal humeral nonunion, thirteen months after an open, extensive elbow injury, can be seen in . Successful treatment of atrophic nonunions require not only removal of interposed scar tissues and adequate stabilization, but also supplemental bone grafting, bone substitutes and/or supplemental bioactive factors.1
Figure 2: (B) PA and (C) lateral radiographs of a distal humeral atrophic nonunion, thirteen-months following open reduction and internal fixation of a comminuted, open distal humerus fracture. (A) Extensive soft tissue damage and periosteal stripping at the time of injury likely contributed to inadequate vasculature and for healing.
Properties of Bone Grafts
As discussed, bone grafts, bone substitutes and bioactive factors foster bone healing through a variety of osteoconductive, osteoinductive and osteogenic mechanisms. depicts the healing properties, functions and costs of various forms of bone grafts.
Table 2: Properties, functions and costs of various forms of bone grafts and substitutes
Osteoconductive properties
Osteoconduction is the process by which an implanted scaffold passively allows ingrowth of host capillaries, perivascular tissue and mesenchymal stem cells (MSCs).Citation5 Microscopically, these scaffolds have similar structure to that of cancellous bone.Citation6 Among the most commonly used osteoconductive scaffolds are calcium sulfate and calcium phosphate cements.
Osteoinductive properties
Osteoinduction is the process by which MSCs from a host are recruited to differentiate into chondroblasts and osteoblasts, which, in turn, form new bone through the process of endosteal ossification. This specialized process is moderated primarily by growth-factors, such as bone morphogenetic proteins (BMP) -2, -4 and -7; platelet derived growth factor (PDGF); interleukins; fibroblast growth factors (FGF); granulocyte-macrophage colony-stimulating factors; and angiogenic factors such as vascular endothelial growth factor (VEGF).Citation5
Osteogenic properties
Osteogenesis is the synthesis of new bone by donor cells derived from either the host or graft donor. Cells involved in this process include MSCs, osteoblasts and osteocytes.Citation5 Only fresh autologous grafts and bone marrow transplants, whether auto- or allo-grafted, are typically involved in this process. Cells recruited for osteogenesis may be transplanted from another site in the body, as is seen with the application of iliac crest bone graft in the setting of a tibial nonunion, or they may be recruited locally, such as calcaneus cancellous harvesting for use in talar neck fracture fixation. Local osteogenic recruitment is the basis behind surgical decortication techniques during bone fusion procedures, whereby cortical bone is removed at the desired site of fusion to expose cancellous bone, which is comparatively rich in osteogenic osteoblasts.Citation5
Autologous Bone Grafts
Autologous bone grafting is the process by which osseous matter is harvested from one anatomic site and transplanted to another site in the same patient. This type of bone grafting is considered the gold standard, as it confers complete histocompatibility while possessing osteoinductive, osteoconductive and osteogenic healing potentials.Citation5 However, autogenous grafting has several limitations related to the harvesting process. These include donor site pain (the most common complication), increased blood loss, increased operative time and the potential for donor site infection.Citation5,Citation7 Additionally, there exists an inherently limited supply of graft—a particular challenge in pediatric patients. The types of autologous grafting commonly used in the setting of nonunion are discussed herein.
Cancellous bone graft
Cancellous bone is the most commonly used form of autologous bone grafting. Its high concentrations of osteoblasts and osteocytes give it superior osteogenic potential. Additionally, its large trabecular surface area encourages revascularization and incorporation at the recipient site.Citation5
Incorporation of cancellous bone grafts is a well-studied phenomenon, characterized by formation of new bone over a necrotic graft bed through the dual processes of resorption and substitution. Following transplantation of a cancellous autograft, a local hematoma forms that is rich in inflammatory cells and chemotactic mitogens and low in oxygen tension, which leads to the further recruitment of MSCs. These cells, in turn, lay down fibrous granulation tissues, typically within 48 hours of injury.Citation8 Macrophages, too, are recruited, and the necrotic graft tissue is slowly removed. During this process, neovascularization is also occurring.Citation5 Next, the autograft is incorporated. Osteoblasts line the periphery of the dead trabeculae and produce osteoid. Osteoid, in turn, is mineralized to form new bone, a process that takes 6 to 12 months following grafting.Citation8
Cortical bone graft
Unlike cancellous bone, whose low density lends little mechanical support, cortical bone possesses excellent structural integrity. However, its dense, highly organized structure comes at the cost of a comparatively limited supply of osteoblasts, osteocytes and other cellular progenitors. This lack of cellularity results in the limited osteogenic and osteoinductive properties of cortical bone grafts.Citation5,Citation9 Further, there is evidence that most osteocytes actually die following transplantation, further degrading its osteogenic potential.Citation9 Given the dense organization of cortical bone, revascularization is hampered. Overall, cortical autograft is much slower to incorporate than cancellous graft.
The process by which cortical bone is incorporated is mediated predominantly by osteoclasts, as opposed to osteoblasts. Incorporation occurs through a process known as creeping substitution, or the slow, near-complete resorption of the graft with simultaneous deposition of new, viable bone.Citation5,Citation7 Creeping substitution begins at the graft-host junction, then moves along the axis of the cortical graft. Depending on the size of the graft and both local and systemic conditions for healing, graft incorporation can take many years to complete. Although necrotic transplanted bone quickly loses strength—up to 75% of initial strength over the resorption period—it eventually heals with minimal-to-no residual weakness.Citation5,Citation7
Vascularized bone graft
The harvesting of vascularized bone graft requires preservation of its nutrient, metaphyseal and/or additional perforating vessels, as these are anastomosed—both arteries and veins—to nearby vessels upon delivery to the recipient site. In vascularized grafts, the graft’s osteocytes and other osteoprogenitor cells are preserved. Viable grafts are incorporated not by creeping substitution, but by primary or secondary bone healing.Citation11 This may increase the graft’s osteogenic potential and prevent the initial loss of graft strength seen with non-vascularized cortical grafts.Citation9 Commonly utilized vascularized grafts include free fibula strut grafts (peroneal artery); free iliac crest grafts (deep circumflex iliac artery branches); and distal radius grafts (1–2 intercompartmental supraretinacular artery).
Bone marrow aspirate
Although less commonly employed than cancellous or cortical grafts, bone marrow aspirate has been shown in several clinical studies to be effective in the treatment of both osseous defects and nonunions.Citation1 This was previously attributed to its high concentration of MSCs and presumed direct osteogenic potential. However, recent studies have shown that the actual concentration of MSCs in bone marrow aspirate is significantly lower than once thought.Citation5 A different, more recent, theory for the effectiveness of bone marrow aspirate in fracture healing pertains to the endothelial progenitor cells it possesses and their ability to stimulate angiogenesis and restoration of blood flow at the fracture site.Citation7 This hypothesis identifies a possible indirect osteoinductive role for bone marrow aspirate.
As a viscous fluid, injected percutaneously at a fracture or nonunion site, marrow aspirate provides no structural support. Further, its liquid form lends itself to seepage from the site of injury. These limitations have led to current research devoted to more effective marrow aspirate delivery via semisolid substrates such as demineralized bone matrix and collagen.Citation5
Platelet-rich plasma
Platelet-rich plasma (PRP) is an autologous suspension of platelets prepared from whole blood via double-centrifugation techniques. The extremely high concentrations of platelets in PRP are rich in several key growth factors. These factors include, but are not limited to: PDGF, transforming growth factor-beta (TGF-β) and VEGF.Citation7 Once extracted from the patient’s blood, platelets are activated with calcium chloride and the resultant platelet clot may be applied to the site of injury. Despite animal research that found PRP enhances cellular proliferation, chondrogenesis and callus strength when delivered to a fracture site, the literature lacks strong evidence to support its routine use in human fracture healing.Citation7
Allopathic Bone Grafts
Allopathic bone graft refers to bone that is harvested from human cadavers, sterilely processed and transplanted to a recipient. Allograft comes in a variety of forms, including osteochondral, cortical, cancellous and highly processed bone derivatives such as demineralized bone matrix (DBX). Depending on the preparation process, allograft exhibits osteoconductive and sometimes osteoinductive potential. In most instances, allografts do not include viable cells and are therefore not osteogenic;Citation9 the major exception, of course, being bone marrow allograft transplant, which, at least today, is seldom employed in fracture healing.
Over 200,000 cases involving allografts are used per year in the US.Citation5 The lack of donor site morbidity, general success of outcomes and decreased surgical times make allograft a popular alternative to autologous bone graft. Yet, high costs and risks such as viral transmission make allograft an imperfect substitute. Rates of viral transmission from orthopaedic allografts are based upon are those of blood products and, fortunately, are exceeding rare. The risk of viral transmission associated with blood properly screened for Hepatitis B one in 63,000, for Hepatitis C it is one in 100,000 and for HIV it is less than one in 1,000,000.Citation10
Allograft preparation involves the removal of soft tissues and cells with ethanol, followed by gamma irradiation for bacterial, fungal and viral sterilization. Unfortunately, irradiation adversely affects the graft’s biologic properties in a dose-dependent manner. High-dose irradiation not only compromises any osteogenic and osteoinductive properties that the allograft may have had, but also leads to polypeptide chain splitting and water molecule radiolysis, the result of which is diminished structural integrity.Citation7
Cancellous allograft
Cancellous allograft is produced in the form of small cuboid chips or “croutons,” so nicknamed for their shape and texture. They are used to pack osseous defects, such as those seen in the setting of total hip arthroplasty associated retroacetabular osteolysis. Like autogenous cancellous grafts, cancellous allografts confer little mechanical strength. Unlike the autografts, however, they are a relatively poor promoter of healing secondary to their preparation process, which leaves them devoid of many of the growth factors that foster osteoinduction.Citation7 In essence, they are an osteoconductive graft.
Unlike autologous cancellous graft incorporation, a local host inflammatory response drives cancellous allograft incorporation, leading to the production of an encapsulated layer of fibrous tissue around the graft. This fibrous tissue makes it more challenging for the host to deposit new osteoid and bone, not only delaying osseointegration for months to years, but also preventing its complete integration.Citation7
Cortical allograft
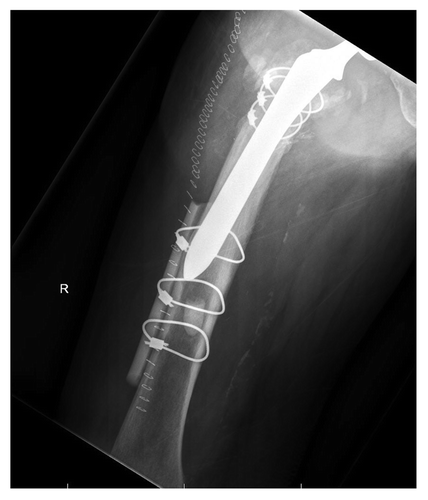
Cortical allografts provide rigid structural support, are readily available and can be used in conjunction with multiple other fracture repair techniques. Like cortical autografts, they can be used to fill large defects, and, depending on the stability of their fixation, allow for early weight bearing even before bony incorporation has occurred. Cortical allografts are a commonly employed in spine procedures, as their resistance to compressive strength allows them to be placed in areas requiring immediate load-bearing resistance. They are also frequently used to provide stabilization to the femoral shaft in the setting of periprosthetic hip fractures. depicts a cortical allograft strut, used to reinforce cable fixation of a periprosthetic hip fracture that occurred at the tip of the femoral implant.
Figure 3: AP radiograph of a periprosthetic femur fracture following cable fixation augmented with a cortical strut femoral allograft.
Cortical allograft incorporation, like cancellous allograft, involves an initial inflammatory cascade. However, after the initial inflammatory stages, its healing is more representative of its autogenous counterpart, with creeping substitution as the key process of incorporation.Citation5
Demineralized bone matrix
Demineralized bone matrix (DBM) is a readily available and popular form of bone graft, comprising approximately 50% of all allografts performed in the US.Citation14 DBM is a popular adjunct to spinal fusion procedures, the grafting of nonunions and the filling of bony defects. It is a form of highly-processed allograft consisting of collagens, non-collagenous proteins, BMPs and other growth factors that bestow it with both osteoinductive and osteoconductive properties.
The osteoinductive properties of DBM are greater than those of cancellous or cortical allografts.Citation7 Its osteoinductive properties stimulate healing through growth-factor-mediated differentiation of MSCs into osteoblasts. DBM’s osteoinductive capacity, however, is greatly dependent on its preparation technique. Techniques involving alcohol, acetic acid, lactic acid and nitric acid have been demonstrated to have a detrimental effect on osteoinductivity.Citation5 Much of the commercially available DBX today is washed with hydrochloric acid. Many modern DBM preparations, too, are admixed with cortical and/or cancellous bone chips to confer additional osteoconductive properties.Citation5
Synthetic Bone Grafts
Synthetic calcium salt-based bone substitutes are an osteoconductive alternative to both autologous and allogenic graft options, given their wide-availability, comparatively low cost and absence of risks such as donor site morbidity and viral transmission. Because they are strictly osteoconductive, their biologic role in fracture healing is limited. Bone substitutes come in a variety forms, including powders, putty, pellets, or as coatings on implants, such as hydroxyapatite-coated joint prostheses. Additionally, they can be mixed with additives such as antibiotics, making them appealing in the setting of infectious-associated osseous defects. Research is underway to test to efficacy of adding collagen, growth factors and even MSCs to these synthetics.Citation7 Such biologically active components may bestow osteoinductive, or even osteogenic properties to these materials. depicts the common physical forms, characteristics and common commercial brands of synthetic grafts.
Table 3: Bone substitutes: properties and commercial product examples
Calcium sulfate
Calcium sulfate is a relatively inexpensive, widely available synthetic bone substitute. It is available in various forms, including hard pellets [Osteoset (Wright Medical Technology), JAX (Smith & Nephew), Calceon (Synthes)] and injectable viscous fluids (MIIG, Wright Medical Technology) that harden in vivo. The obvious advantage of liquid graft application is that it allows for the percutaneous filling of bone voids. Calcium sulfate grafts are the most rapidly absorbed synthetic bone substitute available. Resorption typically occurs in one to three months—a faster rate than actual bone deposition.Citation11 The true clinical significance of such mismatched processes is yet to be established.
Calcium phosphate
The calcium phosphate synthetic substitutes are a family of calcium salt compounds consisting of varying proportions of calcium ions and organophosphates. Calcium phosphate compounds used in bone grafting include monocalcium phosphate, dicalcium phosphate, tricalcium phosphate, hydroxyapatite (both alpha- and beta-crystalline forms) and tetracalcium phosphates. Of note, hydroxyapatite is a naturally occurring mineral that comprises almost 50% of bone by weight. Few calcium phosphate cements are available as pure, single-compound formulations; most commercially available products contain varying concentrations of mixed calcium salts. Norian (Synthes), for example, is a popular bone substitute with the mineral composition: α-tricalcium phosphate (85%), CaCO3 (12%) monocalcium phosphate dehydrate (3%).Citation12
Despite their varying mineral compositions, calcium phosphate substitutes share several characteristics that make them a popular choice for many surgeons: slow biodegradation, superior strength in compression and the unique capacity for osteointegration, or the ability for growing host bone to interdigitate with a rough crystalline graft interface. Norian, like many calcium phosphate cements, claims strength of “4–10 times greater than the compressive strength of cancellous bone.”Citation13 While this may be true, the actual in vivo-strength of these compounds is likely inferior to normal cancellous bone. This is because ceramic cements are generally very brittle when subjected to tensile or shear forces, which occur in addition to compressive forces in vivo.Citation12
Orthobiologic Factors
Osteogenic growth factors
Synthetic bone substitutes have been used for decades to facilitate bone healing through osteoconductive means, yet only relatively recently have allopathic, xenomorphic, or synthetic bioactive molecules been utilized to augment fracture repair. The principle of osteoinductive healing is based upon the presence of local growth factors at the site of bone injury. Common examples include bone morphogenetic proteins (BMPs), transforming growth factor-beta proteins (TGF-β), platelet derived growth factor (PDGF), vascular endothelial growth factors (VEGF), among many others. A selection of commonly discussed endogenous growth factors, their known functions and potential orthopaedic indications are presented in .
Table 4: Select endogenous growth factors and their known functions in fracture healing
Bone Morphogenetic Proteins
Since their discovery almost 50 years ago, BMPs have become an intense subject of orthopaedic research, so driven over the past few decades by their evolving orthopaedic indications, promising clinical outcomes, and, of course, ties to a growing industry netting billions of US dollars.Citation14,Citation15 Of the more than twenty identified BMPs, all but one (the exception being BMP-1, a metalloproteinase) are members of the TGF-beta superfamily, a class of closely-related biologic molecules thought to function in complex signaling pathways of osteoblastic differentiation and osteogenesis.Citation7 depicts the known functions, properties and indications for several well-studied bone morphogenetic proteins.
Table 5: Select Bone morphogenetic proteins: properties, indications and product examples
Today, several commercially available BMP products are manufactured in mass quantities and in pure concentrations via molecular cloning techniques and recombinant expression.Citation16 Such techniques involve the transplantation of human BMP genes into bacterial cells which are regulated to produce the protein products; this process is denoted by “rh”-BMP, or recombinant human BMP.
Among their various functions, many of which are still being discovered, several types of BMPs have been shown to possess osteoinductive activity, promote cartilage formation, promote angiogenesis and even serve as biological markers and tumor-suppressors for various cancers of the gastrointestinal system.Citation17,Citation18 Specifically, BMP-2, -4, -6, -7, -9 and -14 have been shown to have significant osteogenic properties.Citation19,Citation20 BMP-2 and -7, additionally, have been demonstrated to directly promote local neovascularization.Citation7
Several unique properties of BMP-2 may account for its robust healing potential. It is the only BMP known to specifically induce osteoblastic differentiation from mesenchymal stem cells.Citation16 rhBMP-2, currently marketed as Infuse (Medtronic Sofamor Danek), is the only US FDA approved BMP approved for insertion within a titanium tapered cage in anterior lumbar interbody fusion (ALIF) procedures.Citation16 Additionally, large multicenter randomized trials supporting the use of rhBMP-2 on long bone fractures have led to their recent FDA approval for use in acute, open tibial fractures.Citation7
BMP-7, also known as human osteogenic protein-1 (OP-1, Stryker Biotech), has similarly been demonstrated to have potent osteoinductive properties as well as a potential to promote angiogenesis. Its use in effective fracture healing has been well-established in multi-center randomized-control trials in which researchers found BMP-7-impregnated collagen carriers to be equally as effective as autologous bone graft (the gold standard) in the setting of tibial non-unions. Evidence of healing in these difficult non-unions exceeded 75% in both control and BMP-7 groups.Citation21 Recently, BMP-7 has been approved as an alternative to autograft settings of long bone nonunions and in patients requiring revision posterior lumbar fusion procedures under the US FDA’s Humanitarian Device Exemption (HDE).Citation7
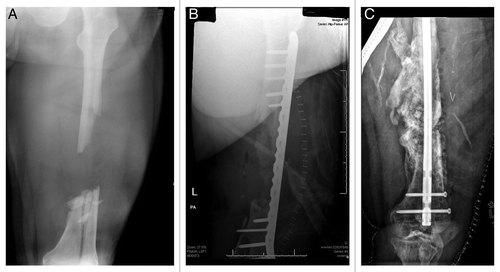
Delivery and containment of BMPs at the fracture site is challenging, and is itself subject to much intensive investigation. BMPs are especially soluble proteins and have a tendency to dissipate from their intended locations, thus diluting their concentrations, and, in turn, their potential effectiveness. Further, there is mounting recent evidence that demonstrate significant morbidities associated with insufficiently contained BMP application. Among these morbidities, providers report BMP-induced ectopic bone formation resulting in painful nerve compressions and ejaculatory dysfunction and intense, life-threatening inflammatory reactions occurring near vital structures of the head, neck and central and peripheral nervous systems.Citation22 demonstrates the remarkable potencies of BMP, used in conjunction with iliac crest autograft and DBM, to fill a large, segmental femoral shaft defect. Ultimately, the expansive bone formation went on to non-union ten months after the bone graft and bridge plate were placed. Successful healing of the now hypertrophic nonunion was achieved with rigid intramedullary fixation.
Figure 4: (A) AP radiograph of a large, segmental femur fracture with a sizeable diaphyseal defect. (B) postoperative AP radiograph of the fracture following bridge plating and implantation of iliac crest bone graph, DBM and BMP. (C) AP radiograph taken ten months following injury showing expansive bone formation and subsequent hypertrophic nonunion following hardware failure, treated with rigid intramedullary fixation.
BMPs require molecular carriers to deliver and maintain them at their intended osseous targets. Such carriers may be structural, i.e. have space-occupying properties (such as synthetic polymers or calcium ceramics) or non-structural, i.e. BMP-soaked collagen sponges. Non-structural carriers are better suited for use inside or around additional implants, such as inter-vertebral body cages in the lumbar spine, or surrounding other implants in long bone defects. Structural carriers are used most commonly when space for future bone growth is desired, such as around the lumbar transverse processes in posterior spinal fusions.
Vascular Endothelial Growth Factor
Vascular Endothelial Growth Factor (VEGF) is a growth factor produced by a various healthy and pathologic cells that stimulates both vasculogenesis, the embryonic development of the circulatory system, as well as angiogenesis, the expansion of blood vessels from existing vasculature. In addition to its various roles in normal physiology and the pathophysiology of illnesses, such as diabetes, respiratory diseases and several forms of cancer, VEGF plays an essential role in fracture healing.
Following fracture hematoma and soft callus formation, VEGF acts on local endothelial cells to induce vascular invasion of the forming hypertrophic cartilage.Citation7 In addition to its initial release by platelets and other inflammatory cells, VEGF has also been found to be secreted from hypertrophic chondrocytes in fracture calluses.Citation7 Vascularization of cartilage is one of the fundamental steps in endochondral ossification, the process by which most growth factors, including BMPs, have been shown to promote bone healing.Citation23
Extra-physiologic concentrations of VEGF have been shown in several independent rodent studies to promote healing of long bone fractures.Citation7 However, little evidence is available thus far of its direct effects on human fracture healing. Of note, high-concentrations of VEGF found in PRP preparations are theorized to contribute largely to the function of PRP.
In addition to VEGF, the most potent and perhaps best-understood angiogenic growth factor, several other growth factors are implicated in the angiogenic processes essential to effective fracture healing. These include fibroblast growth factors, matrix metalloproteinases (MMPs) and, as discussed, BMP-2 and BMP-7. In animal models, FGF has also been shown to enhance local cartilage formation when administered in low doses (Flynn).Citation7 Clinical data for FGF in humans is limited at this time.
Parathyroid Hormone
Parathyroid hormone (PTH) is a well-described endocrine mediator of calcium and phosphate regulation in humans. Pulsatile, low-dose, exogenous administration of PTH has been demonstrated to have anabolic effects on bone metabolism. Teriparatide, a recombinant human parathyroid hormone (rhPTH), is an FDA-approved drug consisting of the first 1-34 amino acid residues of human PTH. In daily pulsatile injections, rhPTH, has been shown to increase bone mineral density in the lumbar spine and femoral necks of patients with osteoporosis, as well as reduce overall fracture risk.Citation7 Its mechanism exploits an imbalance between osteoblast-mediated bone deposition and osteoclast -mediated resorption, with an overall net gain in bone mass.Citation24 Logically, similar imbalanced proportions of bone deposition are desirable in the setting of healing fractures and bony defects.
Early animal studies demonstrate that PTH derivatives may enhance fracture healing. PTH derivatives have been demonstrated to increase both the quantity of, and rate at which, callus, cartilage and thus bone formation takes place. The process of bone remodeling and the strength of formed callus, too, may also be enhanced.Citation7 Additionally, rhPTH may also have true osteoinductive properties, conferred through its effect on the expression of RUNX2 and OSX, two genes known to promote osteoblast differentiation. Indeed, there is some evidence that MSCs undergo accelerated differentiation to maturate osteoblasts in the presence of rhPTH.Citation23 As an increasingly popular treatment for recalcitrant osteoporosis, further clinical research will determine the role of rhPTH in the enhancement of fracture healing.
Costs
More so today than ever before, a careful consideration of the advantages and disadvantages of bone grafts would be incomplete without discussion of their costs. In a 2008 review, Mendenhall Associates, Inc. and the Orthopaedic Network News organization presented purchasing data for bone grafts from several hundred US hospitals over the four year period, 2004–2007. They determined the average selling prices for several of the leading brand bone grafts and graft substitutes and estimated the average quantities of bone graft used per surgical procedure.
Bone substitutes, including calcium-based ceramics with or without organic additives were used in over 50% of bone-graft cases, with DBM about 33% of cases and allografts (including cortical and cancellous derivatives, but not DBM) in 15% of cases. Over half of cases employing DBM used either the DBX (Synthes/MTF) or Grafton (Osteotech) products. On average, approximately 7 mL of DBM was required per-case, costing an average of approximately $750 per case, or $107 per mL used.Citation14 The most popular synthetic ceramics used were Mastergraft (Medtronic Sofamor Danek) and Vitoss (Orthovita), which together, comprised more than half the bone graft substitute market. In cases in which synthetic grafts were used, surgeons applied an average of 12.7 mL of graft at an average price of approximately $1,100 per case, or $87 per milliliter.Citation14
The majority of bone allografts in the US are distributed through the Musculoskeletal Transplant Foundation (MTF), which manages about 85% of the market.Citation14 Prices for allografts, taken from hospital purchasing data from the Hospital for Special Surgery, show MTF cancellous chips to cost around $376-396 per 30 mL vials, or $13 per milliliter. Femoral shaft cortical grafts, ranging from 3-20cm in length cost from $530–$1,681.Citation24
Prices for BMP-7 (OP-1; Stryker) are approximately $5,000 per one time use. BMP-2 (Infuse, Medtronic Sofamor Danek) costs from $3,500-4,900, depending on the quantity used.Citation25
With these reported figures exists several caveats, namely, (1) these data are from the early-to-mid 2000s (2) the products come in prepackaged sizes, so although an average of 12.7 mL of calcium synthetic grafts were used per case, these products typically come in 5, 10, 15 and 20 mL packages, leading to inevitable wasted material and (3) approximately 80% of the cases from which this pricing data was acquired involved spine procedures, the majority of which were elective cervical and lumbar fusions and therefore do not represent the same populations as the subject of this paper, bone grafting in traumatic skeletal injuries. Finally, and perhaps most importantly, raw material pricing does not take into account the efficacy and thus true cost-effectiveness of these products. BMPs are often criticized for their high prices, yet at even $5,000 per use, they remain significantly less costly than a potential revision surgery. Multiple papers have been published on the true cost-effectiveness of BMPs, yet, no obvious consensus has been reached.Citation16 Providers must use discretion and a careful weighing of the pros and cons when utilizing these powerful, but often double-edged tools.
Conclusion
Effective bone healing requires essential elements: a nutritious and mechanically sound environment, availability of progenitor cells and presence of the various growth factors that trigger their productivity and recruitment. Understanding the concepts of osteoconduction, osteoinduction, osteogenesis are essential to the practicing orthopaedist, whose judicious of bone graft, bone substitutes and bioactive factors help guide the skeleton’s remarkable regenerative potential.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kwong FNK, Harris MB. Recent developments in the biology of fracture repair. J Am Acad Orthop Surg 2008; 16:619 - 25; PMID: 18978283
- Fayaz HC, Giannoudis PV, Vrahas MS, Smith RM, Moran C, Pape HC, et al. The role of stem cells in fracture healing and nonunion. Int Orthop 2011; 35:1587 - 97; http://dx.doi.org/10.1007/s00264-011-1338-z; PMID: 21863226
- Russell AT, Taylor CJ, Lavelle DG. (1991) Fractures of tibia and fibula. In: Bucholz RW, Heckman JD, Court-Brown CM (eds) Fractures in Adults, Rockwood and Green, vol 3. pp 1915– 1982.
- Taylor CJ. (1992) Delayed union and nonunion of fractures. In: Crenshaw AH (ed) Campbell’s Operative Orthopaedics, vol 28.Mosby, pp 1287—1345.
- Khan SN, Cammisa FP Jr., Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg 2005; 13:77 - 86; PMID: 15712985
- McKee MD. Management of segmental bony defects: the role of osteoconductive orthobiologics. J Am Acad Orthop Surg 2006; 14:10 Spec No. S163 - 7; PMID: 17003191
- Flynn JM. “Fracture Repair and Bone Grafting.” OKU 10: Orthopaedic Knowledge Update. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2011. 11-21.
- Oakes DA, Cabanela ME. Impaction bone grafting for revision hip arthroplasty: biology and clinical applications. J Am Acad Orthop Surg 2006; 14:620 - 8; PMID: 17030595
- Bae DS, Waters PM. Free Vascularized Fibula Grafting: Principles, Techniques, and Applications in Pediatric Orthopaedics. Orthopaedic Journal at Harvard Medical School 2006; 8:86 - 9
- Laurencin CT. “Musculoskeletal Allograft Tissue Banking and Safety.” Bone Graft Substitutes. W. Conshohocken, PA: ASTM International, 2003; 30-67.
- Hak DJ. The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg 2007; 15:525 - 36; PMID: 17761609
- Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater 2010; 20:1 - 12; PMID: 20574942
- Synthes International. Advertisement. Norian SRS. N.p., n.d. Web. 7 Oct. 2012. <http://www.synthes.com/sites/intl/Products/Biomaterials/Trauma/Pages/Norian_SRS.aspx>.
- Mendenhall S. “Bone Graft and Bone Substitues.” Orthopedic Network News. Mendenhall Associates, Inc., 10 Nov. 2008. Web. 30 Sept. 2012. <http://old.orthopedicnetworknews.com/>.
- Obremskey WT, Marotta JS, Yaszemski MJ, Churchill LR, Boden SD, Dirschl DR. Symposium. The introduction of biologics in orthopaedics: issues of cost, commercialism, and ethics. J Bone Joint Surg Am 2007; 89:1641 - 9; http://dx.doi.org/10.2106/JBJS.F.01185; PMID: 17606804
- Rihn JA, Gates C, Glassman SD, Phillips FM, Schwender JD, Albert TJ. The use of bone morphogenetic protein in lumbar spine surgery. J Bone Joint Surg Am 2008; 90:2014 - 25; PMID: 18762664
- Milano F, van Baal JW, Buttar NS, Rygiel AM, de Kort F, DeMars CJ, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology 2007; 132:2412 - 21; http://dx.doi.org/10.1053/j.gastro.2007.03.026; PMID: 17570215
- Kodach LL, Wiercinska E, de Miranda NF, Bleuming SA, Musler AR, Peppelenbosch MP, et al. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology 2008; 134:1332 - 41; http://dx.doi.org/10.1053/j.gastro.2008.02.059; PMID: 18471510
- Even J, Eskander M, Kang J. Bone morphogenetic protein in spine surgery: current and future uses. J Am Acad Orthop Surg 2012; 20:547 - 52; http://dx.doi.org/10.5435/JAAOS-20-09-547; PMID: 22941797
- Khan SN, Fraser JF, Sandhu HS, Cammisa FP Jr., Girardi FP, Lane JM. Use of osteopromotive growth factors, demineralized bone matrix, and ceramics to enhance spinal fusion. J Am Acad Orthop Surg 2005; 13:129 - 37; PMID: 15850370
- Friedlaeder GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, et al. Osteogenic Protein- (Bone Morphogenetic Protein-7) in the Treatment of Tibial Nonunions. The Journal of Bone and Joint Surgery 2001; 83-A:Supplement 1, Part2 151 - 8; PMID: 11712836
- Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 2009; 302:58 - 66; http://dx.doi.org/10.1001/jama.2009.956; PMID: 19567440
- Einhorn TA, Lee CA. Bone regeneration: new findings and potential clinical applications. J Am Acad Orthop Surg 2001; 9:157 - 65; PMID: 11421573
- Kaback LA, Soung Y, Naik A, Geneau G, Schwarz EM, Rosier RN, et al. Teriparatide (1-34 human PTH) regulation of osterix during fracture repair. J Cell Biochem 2008; 105:219 - 26; http://dx.doi.org/10.1002/jcb.21816; PMID: 18494002
- Bostrum MPG, Seigerman DA. The Clinical Use of Allografts, Demineralized Bone Matrices, Synthetic Bone raft Substitutes and Osteoinductive Growth Factors: A Survey Study. The Hospital for Special Surgery Journal 2005; 1:9 - 18
- "BMP1 Bone Morphogenetic Protein 1." National Center for Biotechnology Information. U.S. National Library of Medicine, 12 Sept. 2012 [updated]. Web. 09 Oct. 2012. <http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=649 >.
- Kan L, Hu M, Gomes WA, Kessler JA. Transgenic Mice Overexpressing BMP4 Develop a Fibrodysplasia Ossificans Progressiva (FOP)-Like Phenotype. Am J Pathol 2004; 165:1107 - 15
- Meynard D, Vaja V, Sun CC, Corradini E, Chen S, López-Otín C, et al. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood 2011; 118:747 - 56; http://dx.doi.org/10.1182/blood-2011-04-348698; PMID: 21622652
- Kumar V, Abbas AK, Fausto N, Aster J. (2009). Robbins and Coltran Pathologic Basis of Disease. Saunders; 8 edition (2009) pp. 88–89.
- Rosenbloom AL. . Insulin-like growth factor-I (rhIGF-I) therapy of short stature. J Pediatr Endocrinol Metab 2008;; 21:301 - 15