Abstract
Adipose tissue contains some populations, adipose-derived stem cells (ADSCs) which can differentiate into adipogenic, chondrogenic, osteogenic, myogenic, and endothelial cells. Furthermore, adipose tissue can be easily obtained in large quantities through a simple liposuction. ADSCs are thought to be an alternate source of autologous adult stem cells for cell-based therapy. However, it is time-consuming and inefficient to harvest ADSCs by using a traditional collagenase-digestion method. To meet the demand of large quantities of ADSCs in the basic and applied research of regenerative medicine, we developed a rapid and efficient method for isolation and culture of primary ADSCs. The results indicated that the ADSCs obtained with our method possessed strong abilities of proliferation and colony formation in vitro, and could keep low level of cell senescence with stable population doubling during long-term culture in vitro. Furthermore, these harvested ADSCs were capable to differentiate into osteogenic and adipogenic lineages in the specific induction medium. In addition, the results of flow cytometry analysis indicated that these ADSCs could positively express multiple CD markers, such as CD44, CD105, CD29, CD90, and CD13, and hardly expressed CD31, CD34, CD45, and CD106, which was homologous to the mesenchymal stem cells. Therefore, the ADSCs isolated with our method are consistent with previously reported characteristics of the ADSCs. This new method that we established in this study is an efficient tool to isolate and culture the stem cells from adipose tissue.
Introduction
Mesenchymal stem cells (MSCs) with self-renewal potential can differentiate into different lineages of tissue types, including cartilage, fat, bone, tendon, skeletal muscle, and ligament in the appropriate microenvironment.Citation1-Citation4 This makes MSCs an attractive source of adult stem cells for cell-based therapies in tissue engineering and regenerative medicine.Citation5-Citation7 In addition, bone marrow, which has a potential limitation due to its limited availability, is the main source of MSCs, although other tissue such as umbilical cord blood, peripheral blood, and placenta also contain some population of MSCs.Citation8-Citation10
Since Zuk and other researchers found that adipose tissue contained an abundant source of adult stem cells,Citation11-Citation13 adipose-derived stem cells (ADSCs) have many potential applications in tissue engineering and regenerative medicine. ADSCs are known to be very similar to MSCs in cell surface antigens,Citation14,Citation15 and possess the properties of multipotent cells that can differentiate into adipogenic, myogenic, chondrogenic, osteogenic, and neuronal lineages.Citation16-Citation18 Because adipose tissue can be more easily obtained with less invasive procedures and patient morbidity than other tissue, it is a more reliable source of adult stem cells with substantial therapeutic potential.Citation19,Citation20 A large number of ADSCs can be harvested after multiple steps of processing and culture. Therefore, ADSCs have been proposed as a promising alternative to MSCs from bone marrow.
The traditional methods for isolation and culture of primary ADSCs have been described in previously published articles.Citation19,Citation21,Citation22 Although these methods were widely utilized with some modifications, they shared some indispensable steps, such as enzymatic digestion with collagenase and multiple steps of centrifugation. It has been noted that many steps and excessive handling in the isolation process of ADSCs easily cause some problems, such as increasing the risk of cell contamination and exhausted labor. Accordingly, in this study we looked at establishing a simple isolation method to obtain large numbers of primary ADSCs. As a result, the ADSCs obtained with our method possessed the consistent characteristics of mesenchymal stem cells, and expressed the similar profile of cell surface antigens as reported previously.Citation23,Citation24 Furthermore, these isolated cells could undergo adipogenic and osteogenic differentiation in vitro.
Results
Rapid isolation and primary culture of ADSCs
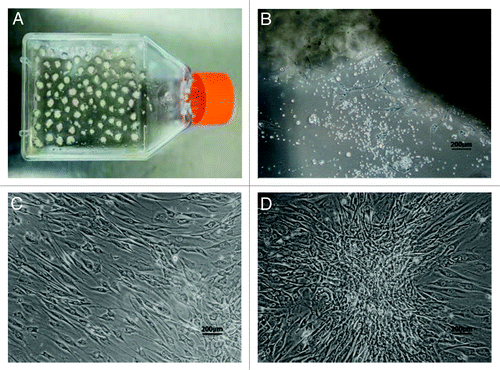
In order to isolate ADSCs, human adipose tissue was cut into pieces that attached to the walls of culture flasks so that stem cells could grow out from the pieces of adipose tissue during the incubation period (). Generally, on the third day of primary culture, many little spot-like cells, defined as ADSCs, appeared from the edges of the pieces of adipose tissue, and they could continue to grow and proliferate. After 7 d of culture, the morphology of ADSCs looked like spindles, and could be easily observed under an inverted microscopy (). After 10–15 d of incubation, the cells reached 80–90% confluence (), and a typical sphere-like colony was observed under an inverted light microscopy (). Subsequently, cell passage was indispensable for ADSCs to expand in vitro. Generally, 1 × 107 ADSCs can be obtained from about 4 g of adipose tissue after 10 d of culture using this method. Therefore, our method is an efficient isolation tool to obtain large numbers of primary ADSCs.
Figure 1. Isolation and primary culture of human ADSCs. The isolated process and primary culture of human ADSCs were described in the Materials and Methods. (A) The pieces of adipose tissue attached on the walls of culture flasks on the first day of culture. (B) The ADSCs demonstrated spindle-like morphology on the seventh day of culture. (C) The ADSCs with fibroblast-like morphology developed into 80–90% confluence after 10 d of primary culture. (D) One representative colony with sphere-like shape was observed in 2-week culture. Scale bars: 200 µm
Cell surface antigens of ADSCs
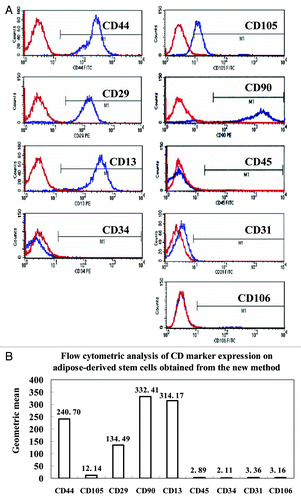
Cell surface markers of ADSCs at the third cell passage were assayed by flow cytometry. The results showed that the ADSCs obtained with our method positively expressed high levels of cell antigens of CD29, CD44, CD90, CD13, and CD105 ( and ), but presented low levels of CD34, CD45, CD31, and CD106 antigens, which were under 4 of geometric mean (). Based on these cell markers, the phenotypes of ADSCs we harvested were almost consistent with previously reported ADSCs obtained by using the traditional collagnase-digestion method.Citation21,Citation22 These results confirm that the cells derived from pieces of adipose tissue were ADSCs, which are homogenous populations of mesenchymal stem cells with no contamination by endothelial cells, pericytes, and smooth muscle cells.
Figure 2. Cell surface antigens of human ADSCs were detected by flow cytometry. The ADSCs at passage 3 were processed with FITC or PE-conjugated monoclonal antibodies to test a set of expression levels of cell surface antigens as described in the Materials and Methods. Nonspecific IgG were examined as a control (red). (A) Representative results of flow cytometry analysis, which showed that ADSCs could expressed positively CD44, CD105, CD29, CD90, and CD13. In contrast, almost no expression of the hematopoietic lineage markers of CD45, CD34, CD31 and CD106 was detected in ADSCs. (B) The geometric mean of cell surface antigens detected by flow cytometry.
ADSCs undergo adipogenic differentiation in vitro
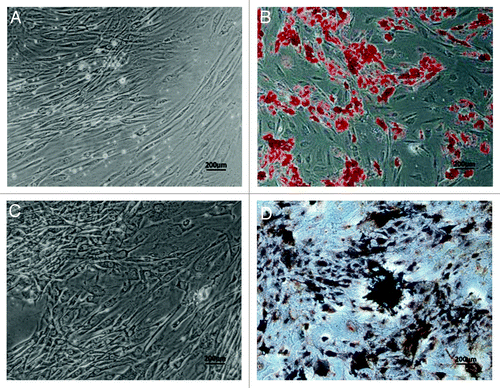
After two weeks of culture in the induction medium, ADSCs presented an expanded cell morphology (), and demonstrated positive staining of Oil red-O solution, indicating the lipid droplets formed on the cytoplasms of cells (). These data confirmed adipogenic differentiation could be induced in our harvested ADSCs.
Figure 3. Adipogenic and osteogenic differentiation of human ADSCs in vitro. Human ADSCs at passage 2 were cultured in the induction medium for two weeks for adipogenic differentiation, or for three weeks for osteogenic differentiation. (A) Adherent ADSCs, no staining as a negative control for adipogenic differentiation. (B) Oil red-O staining for induced ADSCs was positive, and presence of lipid droplets demonstrated that ADSCs could differentiate into adipogenic lineage. (C) Adherent ADSCs, no staining as a negative control for osteogenic differentiation. (D) Von Kossa staining showed that a calcified extracellular matrix of induced ADSCs was detected, which confirmed ADSCs could differentiate into osteogenic cells. Scale bars: 200 µm
ADSCs undergo osteogenic differentiation in vitro
To confirm the osteogenic differentiation of ADSCs, cells were cultured in the induction medium as described in the Materials and Methods. One of the indicators of osteogenic-specific markers is secretion of the collagen I-rich extracellular matrix that becomes calcified at the late stage of differentiation. Therefore, extracellular matrix calcification was evaluated by von Kossa staining. The results showed that ADSCs demonstrated spindle-like cell morphology after three weeks of induction (). Several black regions within the cell monolayers, served being indicative of calcification of extracellular matrix, could be observed in the induced cells (), which implied that osteogenic lineage of these ADSCs could be successfully induced in vitro.
The growth curve of ADSCs
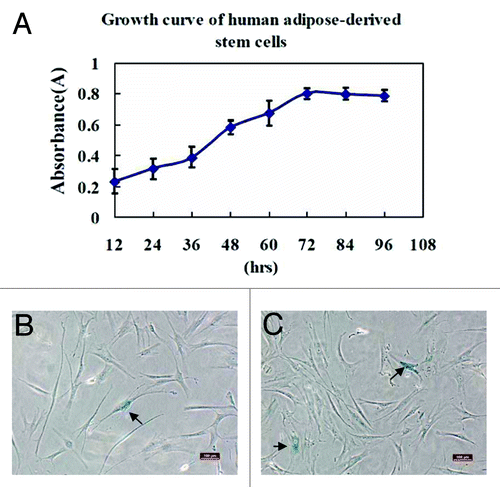
To detect the proliferative potential of ADSCs in vitro, the cells at passage 3 (P3) were seeded in 96-well microplates. Following the culture, cell counting kit-8 was used to examine the absorbance of each well at 450 nm with a microplate reader at indicated time point. The measured data were delineated as a growth curve of ADSCs (), which indicated the proliferative potential of ADSCs could be maintained during extended culture periods
Figure 4. Growth kinetics and senescence of human ADSCs were assayed. The human ADSCs at passage 3 were inoculated in 96-well microplates with 100 μL of cell suspension at a density of 2000 cells/well, and its proliferative potential was measured using cell counting kit-8. (A) The results of cell proliferation were delineated as a growth curve, which showed ADSCs could be amplified and maintained for an extended culture periods. (B and C) Cellular senescence was assayed by staining ADSCs at passage 8 and 15 for β-Gal expression, respectively. The arrows in the photographs indicated β-Gal staining positive cells that were senescent cells, and percentage of cellular senescence at passage 8 (B) and 15 (C) was both below 5%. These results demonstrated that ADSCs could maintain a stable long-term culture period without senescence in vitro. Scale bars: 100 µm
Cell senescence assay
The senescence of ADSCs in long-term culture (passage 3, 8, and 15) was assayed by β-Gal staining which was negative in proliferating cells, but presented blue color in aging cells. The results indicated that cells at passage 3 did not show apparently positive β-Gal staining, although an increase of β-Gal staining was exhibited at later passage 8 and passage 15 (), but the percentage of senescent cells still remained below 5% even at passage 15. Therefore, these results showed that ADSCs could maintain a stable long-term culture period and cell population doubling, keeping a very low percentage of senescence.
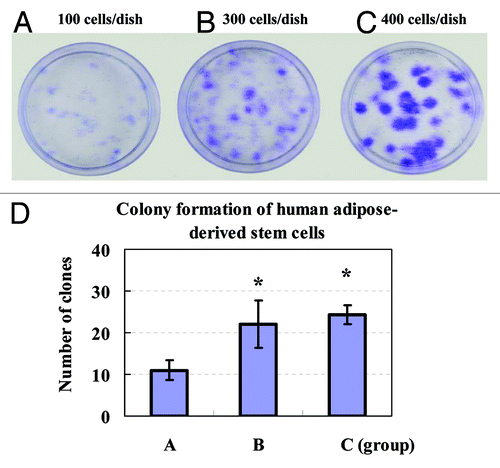
Colony formation of ADSCs in vitro
Clonogenic ability of ADSCs in vitro was detected by crystal violet staining. A colony forming unit (CFU) containing at least 10 cells was defined as a colony. The results showed that colony formation of ADSCs increased in a cell density-dependent manner during the same culture period ().
Figure 5. Colony formation of human ADSCs was assayed. The clonogenic ability of primary ADSCs on the sixth day was analyzed by seeding cells in 35 mm dishes at an indicated cell density for two weeks of culture. Formed colonies were stained with crystal violet solution. Colony forming unit (CFU) containing at least ten cells was defined as a colony and counted under a microscope. (A) 100 cells/dish; (B) 300 cells/dish; (C) 400 cells/dish; (D) The quantities of CFU showed that colony formation of ADSCs increased in a cell concentration-dependent manner. Values were presented as means ± SD (n = 3). *P < 0.05 group B or C vs. group A.
Discussion
Increasing studies have indicated that ADSCs have potential clinical application nowadays. Therefore, an efficient method for isolation and culture of large numbers of stem cells from adipose tissue needs to be developed. In the present study, we report a rapid and efficient method for obtaining primary ADSCs from human fresh sub-abdominal adipose tissue excised surgically. The results indicate large numbers of ADSCs can be obtained with this method from human adipose tissue, and furthermore, the morphology () and surface antigens () of these harvested ADSCs are consistent with previously reported articles.Citation25,Citation26 In our experiments, we found that ADSCs isolated with our method positively expressed CD44, CD105, CD29, CD90, and CD13, although the geometric mean (12.14) of CD105, showed in , was relatively lower than that of CD44, CD29, CD90, and CD13. However, this is consistent with Dr Zuk’s previous reports,Citation27 which indicated that the geometric mean (8.39) of CD105 was also relatively lower than that of CD44, CD90, CD4d, and CD13. On the other hand, the expression of hematopoietic lineage markers CD31, CD34, and CD45 was hardly observed in the ADSCs obtained with our method, which suggest the ADSCs isolated with our method were relatively pure populations of stromal stem cells. In addition, although the surface immunophenotype of ADSCs resembles that of bone marrow-derived mesenchymal stem or stromal cells (MSCs), small differences in surface antigens have been noted between ASDCs and MSCs. Moreover, differences in antibody sources used in different labs could contribute to some discrepancies in detection of cell surface antigens. In addition, these ADSCs obtained with our method could differentiate into adipogenic and osteogenic lineages by induction medium, which indicated these cells at least possessed bipotent properties. Furthermore, these ADSCs had strong clonogenic ability in vitro, and maintained very low percentage of cell senescence during a relatively long-term culture and passages. It is essential to note that there is no use of centrifugation and collagenase digestion in our method, as shown in . In the experiments, we cut adipose tissue into 1–3 mm3 pieces, and placed them into the plastic flasks for culture with suitable amount of FBS. After about 10 d of culture, cells could grow into 80–90% confluence. Due to reduction of multiple steps, such as centrifugation and collagenase digestion, this method undoubtedly decreases intensive labor and the risk of cell contamination in the whole isolation processes, and also saves time and cost. Accordingly, it is a rapid and efficient method to obtain large numbers of ADSCs from adipose tissue. Traditional collagenase-digestion to isolate ADSCs has been adopted in the past decades, but it requires relatively large quantities of adipose tissue (~250 mL) by lipoaspirate.Citation28 Otherwise, few ADSCs can be harvested, because of loss of some stem cells during centrifugation in isolation process. However, large numbers of ADSCs can be produced from relatively less quantities of adipose tissue with our method. About 1 × 107 cells can be obtained from about 4 g of adipose tissue with our method after 10 d of culture. Furthermore, it only needs to take about 1 h to accomplish some simple isolation steps, such as washing, cleaning and cutting adipose tissue into pieces, before starting culture in an incubator at 37 °C. Therefore, our presented isolation protocol is an efficient method to obtain large quantities of ADSCs. Additionally, a possible injury on stem cells in adipose tissue might be avoided because there is no digestion with collagenase during our isolation procedures. In addition, owing to lack of spin in our isolation process, loss of stem cells can be rescued.
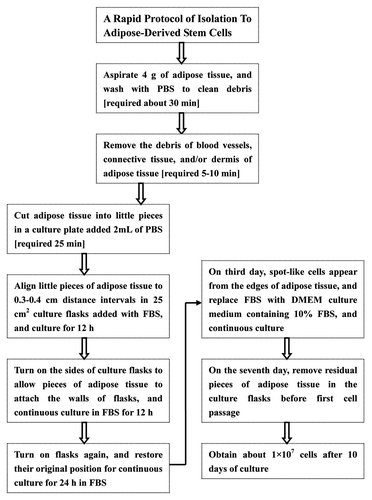
Figure 6. Isolation procedure of human ADSCs with technique flowcharts. No centrifugation and collagenase digestion are required in our isolation protocols for ADSCs from human adipose tissue, and multiple steps and intensive efforts are also decreased, but large numbers of ADSCs can be harvested by this new method.
In order to obtain high quality and large quantities of ADSCs, we found that use of 100% FBS at the early stage of primary culture was very important for ADSCs to come out from pieces of adipose tissue (). We speculated that 100% FBS could offer more growth factors and nutrition to support stem cells in adipose tissue to grow more rapidly than general culture medium with 10–20% FBS. Besides, 100% FBS could help pieces of adipose tissue firmly attach the walls of plastic flasks before stem cells coming out from adipose tissue. If 100% FBS was replaced with general culture medium with 10–20% FBS at the early stage of primary culture, pieces of adipose tissue attached the walls of culture flasks might easily float up in the culture medium before stem cells grow out. Once pieces of adipose tissue drifted up in the culture medium at the early stage of culture, no stem cells could grow out from the adipose tissue. Therefore, using suitable quantities of 100% FBS at the early stage of primary culture plays an important role in strengthening the attachment of pieces of adipose tissue on the walls of plastic flasks. Generally, 2 mL of 100% FBS is suitable to culture pieces of adipose tissue in 25 cm2 culture flask, and help them to attach the walls of plastic flask. In addition, 100% FBS was replaced by DMEM-Low glucose medium supplemented with 10% FBS on the third day of primary culture, and at that time, ADSCs had already sprouted out from pieces of adipose tissue. Short-term use (about three days) of 100% FBS at the early stage of primary culture could not accelerate cellular senescence (data not shown), although FBS had been thought to contain many growth factors acting as predominantly on the proliferation and differentiation of stem cells.Citation29,Citation30 In this study, our data indicated that ADSCs harvested could undergo adipogenic and osteogenic differentiation in specific induction medium (), which demonstrated their multipotentiality of stem cells. The β-Gal staining revealed ADSCs could keep a very low percentage of senescence during a long-term culture periods in DMEM-Low Glucose medium supplemented with 10% FBS (). Low percentage of cellular senescence is in favor of maintaining the stemness and self-renewal ability of ADSCs. Our data also showed a strong clonogenic ability of ADSCs (). These results suggest that the ADSCs obtained with our method have very good cell viability and quality. In addition, we found that ADSCs were easier to be isolated and obtained from adipose tissue near fascias and blood vessels than that far from fascias and blood vessels (data not shown). We suspect that adipose tissue near fascias and blood vessels might contain more stem cells including ADSCs, and these locations are like a reservoir of stem cells which probably come from bone marrow via blood stream because of much similar characteristics between ADSCs and bone marrow-derived MSCs. However, this hypothesis needs to be confirmed in further research.
In conclusion, we established a rapid and efficient method for isolation and culture of human ADSCs in this study. The ADSCs obtained with our method could differentiate into adipogenic and osteogenic lineages in vitro, and expressed similar cell surface antigens with bone marrow-derived mesenchymal stem cells. In addition, these ADSCs had strong capacities of colony formation and proliferation, and could keep low levels of cell senescence during long-term culture and propagation in vitro. Therefore, our method is an efficient and strong tool with a potential application to isolate and harvest human ADSCs for the application in regenerative medicine.
Materials and Methods
Isolation and culture of human primary ADSCs
Human adipose tissue was obtained from three healthy female donors with a mean age of 15 y old. Informed consent was obtained from each donor, and this study was approved by the Human Research and Ethical Committee of the Hospital. About 4 g subcutaneous adipose tissue were taken from sub-abdominal region of each donor undergoing surgical procedures under local anesthesia. The isolation procedure of ADSCs was showed in with technique flowchart. The fresh adipose tissue taken separately from 3 donors were immediately washed extensively with sterile phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4, pH 7.4) to clean the surface of adipose tissue, blood clots, red blood cells, and local anesthetics. Adipose tissue was then placed into 20 mL of PBS supplemented with penicillin (600 U/mL) and streptomycin (300 mg/mL) in a 10 cm culture plate (Corning) for about 5–10 min at room temperature to remove the debris of blood vessels, connective tissue, and/or dermis of adipose tissue with ophthalmic scissors and forceps. After washing and cleaning again, the adipose tissue was transferred to another 10 cm culture plate added with 2 mL PBS, and was cut into irregular little pieces (1–3 mm3) with a pair of scissors. These little pieces of adipose tissue were immediately pipetted into 25 cm2 culture flasks (Corning) and were aligned to 0.3–0.4 cm distance intervals where stem cells would spread and grow from the pieces of adipose tissue. Subsequently, 2 mL of FBS (Gibco) were added into the culture flasks to nourish the pieces of adipose tissue for 12 h in an incubator at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. Then culture flasks were turned on their sides to allow pieces of adipose tissue to attach to the walls of culture flasks, and continued to incubate for another 12 h in an incubator. Subsequently, the culture flasks were turned again and restored to their original position for continuous culture of 24 h. Replacement of FBS in culture flasks with culture medium for the first time depends on the growth and progress of ADSCs from pieces of adipose tissue. Generally, we first replaced the initial FBS in the culture flasks on the third day of culture with DMEM-Low Glucose medium (Gibco) supplemented with 10% FBS while the ADSCs grew out from pieces of adipose tissue. On the seventh day of culture, culture flasks were washed two or three times with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4, pH 7.4) to remove residual adipose tissue. The culture medium was regularly changed every two or three days with DMEM-Low Glucose medium supplemented with 10% FBS. When primary cells grew into 80–90% confluence, they were digested with 0.25% trypsin containing 0.02% EDTA, and passed for cell continuous growth and proliferation for subsequent experimental assays.
Flow cytometry assay for cell surface antigen
Flow cytometry assay was performed according to a reported method with minor modification.Citation31 Briefly, cell suspension of ADSCs at passage 3 (P3) harvested using 0.25% trysin/EDTA was centrifuged at 800 × g for 5 min, and cells were then fixed with 75% ethanol on ice for 30 min. Cells were then washed with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4, pH 7.4) containing 0.5% bovine serum album, and were filtered through a 250 μm (pore size) nylon membrane. A total of 1 × 106 ADSCs were incubated for 30 min with fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated monoclonal antibodies: CD44, CD45, CD105, CD29, CD34, CD90, CD13, CD106, and CD31 (BD Bioscience) to test cell surface antigen expression. A nonspecific phycoerythrin-conjugated IgG was used to assess background fluorescence for ADSCs staining. CD surface antigens were analyzed on a Becton Dickinson FACSCalibur flow cytometer (Becton-Dickinson) by using CELLQestPro acquisition software.
Adipogenic differentiation in vitro
Adipogenic induction of ADSCs in vitro was followed by the reported procedures with slight modifications.Citation32 Briefly, ADSCs were expanded in 35 mm culture plates (Corning) until passage 2 (P2) at 2 × 104 cells/cm2, and then were induced for two weeks in an adipogenic induction medium, namely DMEM-low glucose culture medium supplemented with 10% FBS, 0.5mM isobutyl-methylxanthine (IBMX) (Sigma-Aldrich), 1 mM dexamethasone (Sigma-Aldrich), 10 mM insulin (Sigma-Aldrich), 200 mM indomethacin (Sigma-Aldrich) and 1% antibiotic/antimycotic solution (Sigma-Aldrich). At the end of two weeks of induction, cells were fixed in 10% formalin for 10 min at room temperature and washed with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4, pH7.4), and stained with 2% (wt/vol) fresh Oil red-O solution (Sigma-Aldrich) for 5 min at room temperature to detect lipid droplets in the induced cells.
Osteogenic differentiation in vitro
Osteogenic induction of ADSCs in vitro was performed according to a reported method with minor modifications.Citation33 The harvested ADSCs at passage 2 (P2) were inoculated at 2 × 104 cells/cm2 in 35 mm culture plates added induction medium consisting of regular DMEM-low glucose culture medium, 10% FBS, 0.1 μM dexamethasone (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), 0.05 mM ascorbate-2-phosphate (Sigma-Aldrich), and 1% antibiotic/antimycotic solution (Sigma-Aldrich). To reveal osteogenic differentiation of ADSCs, the cells induced for three weeks were stained with von Kossa kit (Merck Chemicals) for calcium phosphate precipitates. Briefly, the cells were fixed with 4% paraformaldehyde for 1 h at room temperature, and then gently washed with deionised water, and incubated for 30 min in a 1% (wt/vol) silver nitrate solution protected from light. Subsequently, the cells were washed 3 times with deionised water, and detected under UV light for 1 h. Finally, the cells were observed and photographs were taken under an inverted microscope (OLYPAS).
Growth kinetics of ADSCs
Cell Counting Kit-8 (CCK-8) was used to assay grow kinetics of ADSCs followed previous procedures.Citation34 Briefly, ADSCs at passage 3 (P3) harvested by centrifugation were inoculated in 96-well microplates with 100 μL of cell suspension at a density of 2000 cells/well. Ten microliters of CCK-8 solution were added to each well of 96-well microplates and incubated for 1 h at 37 °C, and subsequently, absorbance of each well at 450 nm was immediately measured with a microplate reader every 12 h after cell seeding until 96 h. The measured values of absorbance at different time point were performed as a growth curve standing for growth kinetics of ADSCs.
Cell senescence assay
β-gal actosidase (β-Gal) staining assay was used to determine cellular senescence as previously reported.Citation35 The ADSCs of passage 3, 8 and 15(P3, P8 and P15) were fixed for 5 min in 2% formaldehyde/glutaraldehyde and incubated according to the instructions of β-Gal staining reaction kit (Mirus Bio). Blue color is present in β-Gal staining at pH 6.0 for senescent cells, but it is absent in proliferating cells. Accordingly, senescent cells can be observed and counted under light microscopy.
Colony formation Assay
Colony formation of ADSCs in vitro was analyzed as described previously.Citation36 Briefly, ADSCs of primary culture on the sixth day were harvested and seeded triply in 35 mm cell dishes at a density of 100, 300 and 400 cells/dish, respectively. After 10–14 d of culture in an incubator, the cells were washed with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4, pH 7.4) and fixed with 75% ethanol for 15 min, and then the cells were stained for 25–30 min with Crystal Violet Staining Solution (2.3% certified crystal violet, 0.1% ammonium oxalate and 20% ethanol) (Sigma-Aldrich). After washed 3 times with deionized water, the cellular colonies formed on the dishes were visualized and counted under a microscope. A cell group with more than 10 cells was defined as a colony forming unit (CFU).
Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical significance was evaluated by a two-tailed Student’s t-test, and statistical analyses were performed using SPSS 17.0. The P < 0.05 was considered statistically significant.
| Abbreviations: | ||
| ADSCs | = | adipose-derived stem cells |
| CCK-8 | = | Cell Counting kit-8 |
| FBS | = | fetal bovine serum |
| MSCs | = | mesechymal stem cells |
Acknowledgments
This work was supported by Guangdong Natural Science Foundation (grant number 9151008002000010), Open Project Program of State Key Laboratory of Meat Products for Security and Production Technology in Xiamen (grant number 2011YXGZ008), and Competitive Distributed Program of Financial Fund of Zhanjian City for Science and Technology (Grant number2060499). We thank the critical reading and English proof-reading of the manuscript by Ms Dusty Foster.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Abudusaimi A, Aihemaitijiang Y, Wang YH, Cui L, Maimaitiming S, Abulikemu M. Adipose-derived stem cells enhance bone regeneration in vascular necrosis of the femoral head in the rabbit. J Int Med Res 2011; 39:1852 - 60; http://dx.doi.org/10.1177/147323001103900528; PMID: 22117986
- Dong Y, Zhang Q, Li Y, Jiang J, Chen S. Enhancement of tendon-bone healing for anterior ligament reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci 2012; 13:13605 - 20; http://dx.doi.org/10.3390/ijms131013605; PMID: 23202970
- Liu S, Shao Y, Lin Q, Liu H, Zhang D. 7,8-Dihydroxy coumarin promotes chondrogenic differentiation of adipose-derived mesenchymal stem cells. J Int Med Res 2013; 41:82 - 96; http://dx.doi.org/10.1177/0300060513476614; PMID: 23569133
- Tsai CC, Huang TF, Ma HL, Chiang ER, Hung SC. Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell Transplant 2013; 22:413 - 22; http://dx.doi.org/10.3727/096368912X656090; PMID: 23006509
- Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009; 27:2624 - 35; http://dx.doi.org/10.1002/stem.194; PMID: 19676124
- Wang S, Qu X, Zhao RC. Mesenchymal stem cells hold promise for regenerative medicine. Front Med 2011; 5:372 - 8; http://dx.doi.org/10.1007/s11684-011-0164-4; PMID: 22198748
- Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, et al. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ 2013; 55:309 - 18; http://dx.doi.org/10.1111/dgd.12049; PMID: 23452121
- Ko E, Lee KY, Hwang DS, Villa P, Fumagalli S, Pischiutta F, et al. Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress. Stem Cells Dev 2012; 21:1877 - 86; http://dx.doi.org/10.1089/scd.2011.0284; PMID: 22066510
- Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract 2012; 98:465 - 73; http://dx.doi.org/10.1016/j.diabres.2012.09.034; PMID: 23026513
- Martini MM, Jeremias TdaS, Kohler MC, Marostica LL, Trentin AG, Alvarez-Silva M. Human placenta-derived mesenchymal stem cells acquire neural phenotype under the appropriate niche conditions. DNA Cell Biol 2013; 32:58 - 65; http://dx.doi.org/10.1089/dna.2012.1807; PMID: 23323927
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7:211 - 28; http://dx.doi.org/10.1089/107632701300062859; PMID: 11304456
- Harris LJ, Zhang P, Abdollahi H, Tarola NA, DiMatteo C, McIlhenny SE, Tulenko TN, DiMuzio PJ. Availability of adipose-derived stem cells in patients undergoing vascular surgical procedures. J Surg Res 2010; 163:e105 - 12; http://dx.doi.org/10.1016/j.jss.2010.04.025; PMID: 20638677
- Baer PC, Döring C, Hansmann ML, Schubert R, Geiger H. New insights into epithelial differentiation of human adipose-derived stem cells. J Tissue Eng Regen Med 2013; 7:271 - 8; http://dx.doi.org/10.1002/term.518; PMID: 22162286
- McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro.. Stem Cells 2006; 24:1246 - 53; http://dx.doi.org/10.1634/stemcells.2005-0235; PMID: 16410391
- Zhu X, Shi W, Tai W, Liu F. The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived Mesenchymal stem cells. Cell Tissue Res 2012; 350:277 - 87; http://dx.doi.org/10.1007/s00441-012-1453-1; PMID: 22661317
- Bayati V, Sadeghi Y, Shokrgozar MA, Haghighipour N, Azadmanesh K, Amanzadeh A, Azari S. The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell 2011; 43:359 - 66; http://dx.doi.org/10.1016/j.tice.2011.07.004; PMID: 21872289
- Yoon IS, Chung CW, Sung JH, Cho HJ, Kim JS, Shim WS, Shim CK, Chung SJ, Kim DD. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J Biosci Bioeng 2011; 112:402 - 8; http://dx.doi.org/10.1016/j.jbiosc.2011.06.018; PMID: 21802988
- Santo VE, Duarte AR, Popa EG, Gomes ME, Mano JF, Reis RL. Enhancement of osteogenic differentiation of human adipose derived stem cells by the controlled release of platelet lysates from hybrid scaffolds produced by supercritical fluid foaming. J Control Release 2012; 162:19 - 27; http://dx.doi.org/10.1016/j.jconrel.2012.06.001; PMID: 22698936
- Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 2009; 52:79 - 86; http://dx.doi.org/10.1007/DCR.0b013e3181973487; PMID: 19273960
- Locke M, Feisst V, Dunbar PR. Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cells 2011; 29:404 - 11; http://dx.doi.org/10.1002/stem.593; PMID: 21425404
- Behr B, Tang C, Germann G, Longaker MT, Quarto N. Locally applied VEGFA increases the osteogenic healing capacity of human adipose derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells 2011; 29:286 - 96; http://dx.doi.org/10.1002/stem.581; PMID: 21732486
- Yang XF, He X, He J, Zhang LH, Su XJ, Dong ZY, Xu YJ, Li Y, Li YL. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci 2011; 18:59; http://dx.doi.org/10.1186/1423-0127-18-59; PMID: 21854621
- Bailey AM, Kapur S, Katz AJ. Characterization of adipose-derived stem cells: an update. Curr Stem Cell Res Ther 2010; 5:95 - 102; http://dx.doi.org/10.2174/157488810791268555; PMID: 19941461
- Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 2008; 45:115 - 20; http://dx.doi.org/10.1016/j.ymeth.2008.03.006; PMID: 18593609
- Gagliardi C, Bunnell BA. Isolation and culture of rhesus adipose-derived stem cells. Methods Mol Biol 2011; 702:3 - 16; http://dx.doi.org/10.1007/978-1-61737-960-4_1; PMID: 21082390
- Yu G, Floyd ZE, Wu X, Halvorsen YD, Gimble JM. Isolation of human adipose-derived stem cells from lipoaspirates. Methods Mol Biol 2011; 702:17 - 27; http://dx.doi.org/10.1007/978-1-61737-960-4_2; PMID: 21082391
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13:4279 - 95; http://dx.doi.org/10.1091/mbc.E02-02-0105; PMID: 12475952
- Francis MP, Sachs PC, Elmore LW, Holt SE. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 2010; 6:11 - 4; http://dx.doi.org/10.4161/org.6.1.10019; PMID: 20592860
- Shetty P, Bharucha K, Tanavde V. Human umbilical cord blood serum can replace fetal bovine serum in the culture of mesenchymal stem cells. Cell Biol Int 2007; 31:293 - 8; http://dx.doi.org/10.1016/j.cellbi.2006.11.010; PMID: 17208468
- Aldahmash A, Haack-Sørensen M, Al-Nbaheen M, Harkness L, Abdallah BM, Kassem M. Human serum is as efficient as fetal bovine serum in supporting proliferation and differentiation of human multipotent stromal (mesenchymal) stem cells in vitro and in vivo. Stem Cell Rev 2011; 7:860 - 8; http://dx.doi.org/10.1007/s12015-011-9274-2; PMID: 21603946
- Martinello T, Bronzini I, Maccatrozzo L, Mollo A, Sampaolesi M, Mascarello F, Decaminada M, Patruno M. Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res Vet Sci 2011; 91:18 - 24; http://dx.doi.org/10.1016/j.rvsc.2010.07.024; PMID: 20732703
- Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res 2012; 347:419 - 27; http://dx.doi.org/10.1007/s00441-011-1306-3; PMID: 22287041
- Dosier CR, Erdman CP, Park JH, Schwartz Z, Boyan BD, Guldberg RE. Resveratrol effect on osteogenic differentiation of rat and human adipose derived stem cells in a 3-D culture environment. J Mech Behav Biomed Mater 2012; 11:112 - 22; http://dx.doi.org/10.1016/j.jmbbm.2011.08.014; PMID: 22658160
- Lu YQ, Lu Y, Li HJ, Cheng XB. Effect of advanced glycosylation end products (AGEs) on proliferation of human bone marrow mesenchymal stem cells (MSCs) in vitro. In Vitro Cell Dev Biol Anim 2012; 48:599 - 602; http://dx.doi.org/10.1007/s11626-012-9551-7; PMID: 23054442
- Li XY, Ding J, Zheng ZH, Li XY, Wu ZB, Zhu P. Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol Med Rep 2012; 6:1183 - 9; PMID: 22923041
- Eom YW, Lee JE, Yang MS, Jang IK, Kim HE, Lee DH, Kim YJ, Park WJ, Kong JH, Shim KY, et al. Rapid isolation of adipose tissue-derived stem cells by the storage of lipoaspirates. Yonsei Med J 2011; 52:999 - 1007; http://dx.doi.org/10.3349/ymj.2011.52.6.999; PMID: 22028166