Abstract
Following recent advancements of stem cell research, the potential for organ regeneration using somatic stem cells as an ultimate therapy for organ failure has increased. However, anatomically complicated organs such as the kidney and liver have proven more refractory to stem cell-based regenerative techniques. At present, kidney regeneration is considered to require one of two approaches depending on the type of renal failure, namely acute renal failure (ARF) and chronic renal failure (CRF).
The kidney has the potential to regenerate itself provided that the damage is not too severe and the kidney’s structure remains intact. Regenerative medicine for ARF should therefore aim to activate or support this potent. In cases of the irreversible damage to the kidney, which is most likely in patients with CRF undergoing long-term dialysis, self-renewal is totally lost. Thus, regenerative medicine for CRF will likely involve the establishment of a functional whole kidney de novo. This article reviews the challenges and recent advances in both approaches and discusses the potential approach of these novel strategies for clinical application.
Introduction
Over the past decade, stem cell research has advanced progressively and significantly. Together with the originally discovered hematopoietic stem cells, other stem cells (or progenitor cells) are now well characterized and include endothelial stem cellsCitation1 and neural stem cells.Citation2 This research has expanded the potential for organ regeneration using stem cells to replace affected tissues. In nephrology, in particular, tissue regeneration is gaining considerable attention as a next generation of therapy for renal failure.
Therapies aiming to promote renal regeneration need first to discriminate between the disease states of acute renal failure (ARF) and chronic renal failure (CRF). ARF is a common disease with disparate etiologies that involve reduced total or partial renal blood flow with resultant ischemic injury.Citation3 An ischemic/reperfusion model has therefore been commonly used as an experimental model of ARF. Although the mortality of ARF is high (50–80%),Citation4 few therapies of proven benefit in the treatment or prophylaxis of active disease exist,Citation5 and stem cell therapy to aid regeneration and organ recovery therefore holds much promise for disease management. In some cases, damaged kidneys can be completely restored in structure and function,Citation3 indicating the remarkable regenerative capacity of kidney following acute ischemic injury.
ARF causes both apoptosis and necrosis of renal tubular epithelial cells. Over time, the injured tubules regenerate through cell proliferation, although the source of cells that repopulate the injured nephron is not clear.Citation3 Tissue regeneration may result from proliferation of surviving dedifferentiated cells, from renal stem cells that reside inside the kidney and migrate to the site of regeneration, or from bone marrow cells that gain access to the injured epithelium and differentiate into mature cells.Citation6 It is believed that all solid organs possess both endogenous and exogenous stem cells. After tissue injury, intrinsic “tissue stem cells” replace damaged tissue as a first line of defense. If the pool of endogenous stem cells is exhausted, exogenous circulating stem cells are signaled to replenish the pool and participate in tissue repair as a backup rescue system.Citation7 Therefore regenerative medicine for ARF aims to discover renal stem cells that can be supplied exogenously when kidney is injured and to identify key molecules that can ‘reprogram’ the quiescent tissue stem cells to participate in renal repair. These aims are being addressed using established technologies in regenerative medicine that have been applied to other organs such as heartCitation8 and vessels.Citation9
CRF is the second type of renal failure to be considered in regenerative medicine. The majority of patients with CRF entering dialysis programs have type 2 diabetes, chronic glomerulonephritis, or hypertension.Citation10 The number of CRF patients requiring dialysis has increased dramatically throughout the world mainly due to the significantly extended acceptance criteria for dialysis that now include more elderly and diabetics patients, and those with other severe comorbidities.Citation11 In addition, long-term replacement therapy with hemo- or peritoneal dialysis has dramatically improved the prognosis for CRF patients. Current trends in the population dynamics of maintenance dialysis show an estimated annual worldwide cost of maintenance CRF therapy at near $75 billion US dollars and the size of this global maintenance dialysis population is expanding at a rate of 7% per year. If this current trend continues, the dialysis population will exceed 2 million patients by the year 2010 and the aggregate cost will be more than $1 trillion USD over the coming decade.Citation12 This will render dialysis impractical in the near future as a therapy choice for CRF patients. Although kidneys may be successfully transplanted, the lack of suitable transplantable organs has prevented kidney transplantation from becoming a practical solution for most cases of CRF. This therefore indicates the need for a new type of therapy, by which patients with CRF may discontinue dialysis, and kidney regeneration holds much hope for this purpose. However, the kidney is anatomically complicated and resident cells must communicate with each other to function. A regenerated whole therapeutic kidney must therefore contain fully organized and orchestrated cells able to fulfill their functions. Thus, in contrast to ARF, regenerative medicine for CRF requires a novel approach for building a functional whole kidney de novo. Current attempts to regenerate kidney combine foreign human mesenchymal stem cells with our background knowledge of kidney organogenesis.
In this article, we review recent findings on renal stem cells and their possible application in therapeutic intervention for ARF, as well as the intensive attempts to build a functional kidney de novo for CRF, which may truly progress the next stage from “regenerative medicine” to “regenerative therapy”.
Regenerative Medicine for ARF
Kidney regeneration for ARF using extrarenal cells.
It was reported that in male patients who received kidney transplants from female donors, Y chromosome-positive tubular cells were observed in kidneysCitation13 and about 1% of tubular cells were Y chromosome-positive after the kidneys had recovered from acute tubular necrosis.Citation14 Ours and other groups also found that bone marrow stem cells can contribute to the formation of kidney cells, including mesangial cells,Citation15,Citation16 tubular epithelial cells, and podocytes,Citation17 which gave rise to the hypothesis that some renal stem cells are resident in and mobilized from bone marrow. Therefore many researchers initially tried to identify renal stem cells extrarenaly within bone marrow or the circulation, and many experiments using bone marrow transplantation of marked donor cells were performed to trace their progenies. Among them, Lin et alCitation18 were the first to report that bone marrow stem cells (RhloLin−Sca-1+c-kit+cells) isolated from male RosaCitation26 mice, which express the LacZ gene ubiquitously, may differentiate into renal proximal tubular cells and contribute to renal tubular regeneration when transplanted into female mice with renal ischemia/ reperfusion injury. Kale and coworkersCitation19 subsequently reported the therapeutic potential of bone marrow stem cell infusion for the treatment of ARF. They introduced an ischemia/reperfusion injury in mice with and without bone marrow ablation, to control for the contribution of bone marrow-derived cells. Renal damage in the mice with bone marrow ablation was much worse than observed in normal mice and this exacerbation was reversed by stem cell transfusion. This report provided the conceptual basis for the development of therapeutic strategies involving exogenous renal stem cells to enhance recovery from ARF. Since then, a numbers of papers have reported using different bone marrow fractions or different experimental models to investigate these potential therapies. These studies generally involved the transplantation of bone marrow cells marked with LacZ, EGFP, or a genetic marker (Y chromosome), and their detection after induction of renal damage. Progeny of these donor cells were then detected using X-gal staining, fluorescence microscopy, or fluorescent in situ hybridization for the Y chromosome, respectively. Renal tubular cells bearing these markers were detected, indicating that some fraction of the transplanted bone marrow cells (i.e., bulk fraction, hematopoietic stem cells, and/or mesenchymal stroma) contributed to the renal regeneration following experimental ARF, and confirming the therapeutic potential of such modalities. More recently, however, Duffield et alCitation20 precisely demonstrated that all of the detection systems used in these earlier studies, although well established, were capable of producing false positives, which could overestimate the contribution of bone marrow-derived cells to the renal repair.
According to these collective data, current stem cell research for ARF has, at least partly, moved from a sole focus on bone marrow stem cells toward intrinsic renal stem cells.
Kidney regeneration for ARF using intrinsic renal stem cells.
Three major ways of isolating tissue stem cells currently exist, based on work with other solid organs. Most conventional methods use cell surface markers that can be expressed in the tissue stem cells. CD133 expression was originally shown in hematopoietic stem and progenitor cells,Citation21 but this marker is also expressed by stem cells of other tissues such as vesselsCitation22 and neurons,Citation23 leading to speculation of CD133 being a universal cell surface marker of tissue stem cell. Recently, CD133-positive cells were identified in human adult kidney,Citation24 in the interstitium of the renal cortex. These human CD133-positive kidney cells differentiated into renal tissue in vivo when injected into immunocompromised SCID mice. In addition, intravenously injected CD133-positive cells appeared to integrate with cells of kidney tubules that had been treated with glycerol to induce injury. These data therefore implicated CD133-positive cells as markers of renal intrinsic stem cells.Citation24
Gene expression profiling of mesenchymal cells from embryonic kidney was also used to identify other potential cell surface markers of renal stem cells.Citation25 Challen et al. found 21 genes that were selectively upregulated in cells destined to differentiate into renal tissue. They highlighted CD24 and cadherin-11 as surface proteins that might be useful for isolating viable progenitor cells from the adult kidney. Cells expressing CD24 were incorporated into newly forming tubules, whereas cadherin-11 was expressed primarily on cells that formed the interstitium. Using CD133 and CD24, Sagrinati et al.Citation26 isolated multipotent progenitor cells with the capability to differentiate in vitro into proximal and distal tubules, osteogenic cells, adipocytes and neuronal cells, and a subset of parietal epithelial cells (PEC) in the Bowman's capsule of adult human kidney. Intravenous injection of CD24+CD133+ PEC into SCID mice with glycerol-induced ARF may regenerate tubular structures in different portions of the nephron and therefore reduce any associated morphological and functional kidney damage.
In a different approach that does not use cell surface markers, Kitamura et alCitation27 attempted to establish renal progenitor cells using microdissection. Segments of nephron were cultured separately and, after simple limiting dilution, the cell line exhibiting the most potent growth was isolated. This cell line (rKS56) had the potential to differentiate into mature tubular cells in vitro thus replacing injured tubules and improving renal function after implantation in vivo. Although this method virtually established a renal stem cell line by chance, it would be interesting to know the cell surface marker profile of these cells with the aim of identifying a universal marker of renal stem cells.
Another established way to identify tissue stem cells is by using the DNA marker BrdU, based on the assumption that tissue stem cells cycle very slowly and differentiate only as demanded by tissue turnover. This technique has been used to identify slow-cycling stem cells in other organs including skin,Citation28 intestine,Citation29 and lung.Citation30 However, these tissues all have a rapid cell turnover and it was thought that this approach might prove less successful in slow-turnover tissues like kidney since many renal residential cells such as podocytes have a slow cell cycle and therefore may be falsely identified as stem cells. Maeshima et al.Citation31 did report some success with renal tissue by injecting BrdU intraperitonealy into adult rats once a day for 7 days, and inducing ischemic/reperfusion injury after 14 days. BrdU-positive cells were observed in the tubules but not in glomeruli and capillary vessels. Quantitative analysis showed a two-fold increase in the number of BrdU-positive cells after reperfusion, suggesting that the majority of proliferating cells in the recovering kidney after renal ischemia were derived from BrdU-positive renal progenitor cells.
More recently, Oliver et al.Citation32 injected three-day-old rats subcutaneously twice a day with BrdU for 3.5 days, and then after two months, localized the BrdU-positive cells. During this stage of rat development, the kidney is still growing and residential cells are proliferating, thus slow-cycling cells should be more easily distinguished from other cells. Surprisingly, there were numerous BrdU-positive cells in the renal papilla and only small numbers in the outer-cortex, mid-cortex, and medulla, where they localized mainly within the interstitial area. The BrdU-positive cells were FACS-sorted; they developed epithelial characteristics in vitro and, in vivo, were shown to migrate and incorporate into mature tubules. Following a transient episode of ischemia, the BrdU-positive cells quickly entered the cell cycle and disappeared from the papilla, implicating these cells in renal repair.Citation32 This study therefore suggested that the renal papilla contains a population of adult kidney stem cells involved in kidney maintenance and repair, although the signals and access pathway by which this happens remain unclear.
Detection of side-population (SP) cells is another common method for identifying stem cells. This technique was first used to obtain an enriched population of hematopoietic cells from adult mouse bone marrow using Hoechst 33342 dye staining and FACS,Citation33 with cells negative for this staining deemed as SP cells. This property is due to the expression of efflux pumps belonging to the ATP-binding cassette superfamily of membrane transporters,Citation34 and confer a survival advantage. Therefore the SP phenotype can be used to purify a stem cell-rich fraction. Although the original study isolated a population of uncommitted hematopoietic stem cells, recent studies revealed that SP cells may populate other organs,Citation35 while maintaining their potential as tissue stem cells.Citation36 To identify renal stem cells using this technique, Hishikawa et al.Citation37 isolated kidney SP cells from two congenital mouse models of renal failure and matched controls. Microarray analysis revealed the gene Musculin/MyoR, which is mainly expressed during muscle development,Citation38 to be highly expressed in SP cells, suggesting it may be used as a marker of renal stem cells. The musculin/MyoR-positive cells were localized in the interstitial space of the kidney and systemic injection of these SP cells demonstrated therapeutic potential in the cisplatin-induced ARF model. Although SP cells may indeed be renal stem cells, it is possible that this therapeutic effect was not due directly to differentiation of the SP cells and integration into injured tubule cells, but an indirect paracrine effect on the growth of surrounding cells via HGF, VEGF, and BMP7, since the number of redistributed SP cells after systemic injection was too small to account for a direct contribution.
A recent study by Lin et alCitation39 demonstrated definitive findings to cement the current consensus. They established chimera mice in which mature renal tubular epithelial cells and their progeny are permanently labeled with EGFP. Following ischemia/reperfusion injury in these mice, EGFP-positive cells showed incorporation of BrdU and expression of dedifferentiation markers, vimentin and Pax2. Furthermore, these cells began to express AQP-3 at the basolateral membrane and ZO-1 in the intercellular junction, showing that dedifferentiated intrinsic tubular cells were redifferentiated into mature tubular cells. These data provided the direct evidence that regenerating-tubule cells are derived from renal tubular epithelial cells. To address the relative contribution of intrinsic versus exogenous populations of cells to the renal repair after ischemic/reperfusion injury, quantitative analyses of regenerating BrdU-positive cells and bone marrow-derived Y-positive cells revealed that 89% of the proliferating epithelial cells originated from the host cells and the remaining 11% originated from donor bone marrow cells. In agreement with the earlier work of Duffield et al.,Citation20 these data demonstrated that extrarenal bone marrow-derived cells can be incorporated into renal tubules after ischemic injury, but that intrarenal cells are the major source of tubular regeneration. Therefore, the current major interest in this field is how to harness these cells for the treatment of renal injury.
Molecules that activate renal stem cells.
According to the findings described thus far, renal stem cells do exist within the adult kidney; however, the question remains as to whether enough stem cells are present for therapeutic kidney regeneration after injury. One way to answer this question experimentally might be to identify molecule(s) that recruit and/or enhance quiescent renal stem cells, particularly for activating renal stem cells in response to renal damage. Growth factors have been suggested by some studies as renotropic factors able to activate resident stem cells, such as hepatocyte growth factor (HGF) accelerating the regeneration of tubular parenchymal components after acute injury.Citation40,Citation41 In particular, growth factors that mediate renal development have been intensively examined because it seems feasible that renal regeneration processes may recapitulate developmental processes, and common factors might be involved.Citation42,Citation43 In fact, during perinatal kidney development, HGF induces branching morphogenesis of the ureteric budCitation44,Citation45 and stimulates epithelial differentiation of metanephric mesenchymal cells.Citation46 In addition, bcl-2, bax and pax-2 genes are important in metanephric and urogenital developmentCitation47–Citation49 and are also reexpressed in proximal tubular cells after acute tubular damage.Citation50,Citation51 These examples are consistent with the hypothesis that during tissue regeneration, a cascade of developmental gene pathways may be reactivated. A number of growth factors participate in renal development,Citation52 and several of these were further identified to be renotropic factors important in tubular regeneration of the kidney, including HGF, epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I), and bone morphogenetic protein-7 (BMP-7).Citation53 In addition, leukemia inhibitory factor (LIF), which is crucial for the conversion of mesenchyme into epithelium during nephrogenesis, participates in renal epithelial tubular regeneration.Citation54 These factors are potent regulators of kidney organogenesis,Citation52,Citation55 and administration of these growth factors promotes tubular regeneration after a variety of insults.Citation53,Citation56 Reciprocally, inhibition of a negative regulator of branching morphogenesis during kidney development, activin A, by follistatin accelerates renal regeneration after renal injury.Citation57,Citation58
This work was further enhanced by a recent finding that the homozygous deletion of Sall1 impairs complete ureteric bud outgrowth and tubule formation in the mesenchyme, implicating this gene in the initial step of mesenchymal-to-epithelial conversion.Citation59 Sall1 is distinct from the known regulators of this process. It is also expressed in the subventricular zone of the central nervous system and progress zones of limb buds, where neural and mesenchymal stem cells resides, respectively, leading to speculation that Sall1 might be associated with stem cells in several organs, including kidney. Subsequently, Osafune et alCitation60 established a novel colony-forming assay system using NIH3T3 fibroblast cells expressing Wnt4 and identified the renal progenitors in metanephric mesenchyme using Sall1 as a marker.Citation60 Only cells strongly expressing Sall1 formed colonies from which a three-dimensional kidney structure was reconstituted in an organ culture setting. This assay system might also be used to identify renal stem cells from adult kidney. Furthermore, Araki et alCitation61 used a differential display technique to identify a novel developmental factor, metanephros-derived tubulogenic factor-1 (MTF-1), which possibly facilitates the development of ureteric bud.Citation61 Further research into these molecules may reveal novel renotropic factors, able to specifically activate tissue stem cells to differentiate into mature cells for therapeutic applications.
An important issue for such therapeutic intervention using renotoropic factors is their optimal temporal and spatial delivery to sites of renal injury, since these agents may have diverse and unwanted effects on nontarget organs. Therefore, a gene/drug delivery system is required that can continuously and site-specifically supply these factors. For this purpose, we previously established a bone marrow reconstitution system, by which mononuclear cells expressing therapeutic genes are continuously supplied from the reconstituted bone marrow,Citation62 conferring long-lasting therapeutic effect for at least four months after the primary bone marrow reconstitution.
In addition to autologous cells, exogenous umbilical cord blood may be used as an alternative source of hematopoietic stem cells for bone marrow reconstitution, since taking stem cells from the bone marrow is highly inconvenient for clinical use.Citation63 We previously confirmed that cord blood-derived CD34+cells can be differentiated into monocyte lineage cells and recruited to the site of injury while maintaining transgene expression,Citation64 demonstrating the value of these cells as a source of hematopoietic stem cells for our gene delivery system without pain or risk to the patients. We believe that such a system would provide the next step in regenerative medicine for ARF.
Regenerative Medicine for Chronic Renal Failure
Attempts to establish whole kidney de novo.
Most of the research on kidney regeneration to date has focused on therapies for ARF and only a small number of groups are working on the application of kidney regeneration for CRF, presumably because of the challenges mentioned above in having to rebuild kidney de novo as a whole organ.
Woolf et al.Citation65 previously reported that the metanephros may continue to grow if it is transplanted into the renal cortex of host mice. The developed transplant contains vascularized glomeruli and mature proximal tubules and possibly possesses the glomerular filtration capacity. Collecting duct-like structures appear to extent from transplant towards the papilla of the host. Although there is no direct evidence that they connect with the host's collecting system and that the transplant functions in a manner similar to native kidney, the basic experiment provides the rational of the utility of metanephros from early embryos being a potential source of transplantable regenerated kidney. Potential problems with this system are the suitability of the renal capsule of dialysis-patients as a transplant site, given that this area has been significantly disrupted including vasculature, and the fact that the space limitation beneath the renal capsule may disturb the growth of transplants. These concerns may be overcome by the system established by Rogers et al.,Citation66 who also used embryonic metanephros as a source of transplantable artificial kidney but transplanted the graft into a host omentum that is not confined by a tight organ capsule and has not been disturbed by dialysis. Embryonic metanephros of rat, mouse, and pig was implanted into the omentum of rat or mouse and examined for the success of transplanting across xenogeneic barriers and the extent of differentiation into functional nephron. This experiment was based on previous studies showing minimal immunogenicity in tissues harvested at earlier gestational stages including metanephros.Citation67 In cases of allotransplantation (rat metanephros to rat omentum), transplants assumed a kidney-like shape in situ of approximately one-third the diameter of native kidney. Histologically, they contained well-differentiated kidney structures. Importantly, this transplant technique can be carried out without immunosuppression. With xenotransplantation, pig metanephros grew and differentiated into renal tissue in the rat omentum, showing glomeruli, proximal tubules, and collecting ducts; however, immunosuppressants were required since without these agents, transplants disappeared soon after transplantation. Interestingly, the grafted pig metanephros was slightly larger in volume (diameter and weight) than a normal rat kidney. Furthermore, the transplanted tissue produced urine and surprisingly, after intact ureteroureterostomy with the ureter of the kidney that was removed, anephric rats started to void and showed a prolonged lifespan.Citation68 This success provides promise of a new and practical therapeutic strategy for CRF, which establishes a functional renal unit by implanting xenometanephros together with immunosuppression.
Dekel and coworkersCitation69 aimed to use metanephros from porcine embryos as it is difficult to obtain sufficient numbers of human embryos and substantial ethical problems exist with the use of human embryonic tissue. Metanephroi were isolated from human and porcine embryos at different stages and implanted under the kidney capsule of immunodeficient mice to differentiate into a functional nephron. Seven to eight-week-old human embryonic metanephroi and porcine embryonic metanephroi at embryonic day 27–28 underwent remarkable growth and produced highly differentiated kidney structures, suggesting that embryonic kidney contains renal progenitor cells with the ability to generate many cell types. Most of the vessels in this developing metanephros were derived from the host and the average levels of urea nitrogen and creatinine were higher in cyst fluid arising from this transplant compared with those found in the sera of the transplanted mice. This indicates that the transplant was functional to filter host blood and produce urine, which was the first demonstration of urine production from an artificial kidney. Considering the current shortage of transplantable kidneys, these data suggests the feasibility of pig kidney precursors as an unlimited source for renal transplantation.
In terms of functional whole kidneys, Chan et al.Citation70 reported the first attempt to establish a functional whole renal unit, by developing a transplantable pronephros in Xenopus. Xenopus presumptive ectoderm, which becomes epidermis and neural tissue in normal development, contains pluripotent stem cells and can be differentiated into multi-lineage tissue cells under particular culturing conditions.Citation71 Chan et al designed conditions for the induction of pronephric tubule-like structures from animal caps, involving a combination of activin and retinoic acid for only three hours. This pronephros-like tissue was transplanted into bilaterally nephrectomized tadpoles to test for functional integrity as a pronephros. Bilateral pronephrectomy induces severe edema in tadpoles due to an inability to excrete internal water, and they die within nine days; transplantation of the pronephros-like unit at least partially corrected the edema and the tadpoles survived for up to 1 month. To our knowledge, this study remains the only one to establish a transplantable functional whole kidney unit in vitro, although the pronephros structure formed is too primitive for any clinical application in humans.
To address the adverse effects of immunosuppressants, Lanza et al.Citation72 attempted to establish a self-kidney unit to eliminate the immune-response problem, and therefore the need for immunosuppression. To generate histocompatible kidney for artificial organ transplantation, they used a nuclear transplantation technique, by which dermal fibroblasts isolated from adult cow were transferred into enucleated bovine oocytes and nonsurgically transferred into progestin-synchronized recipients. After 6–7 weeks, metanephroi were isolated from embryos, digested using collagenase, and expanded until the desired cell number was obtained by culturing in vitro. These cells were then seeded on a specialized polymer tube followed by implantation into the same cow from which the cells were cloned. Strikingly, this renal device seeded with cloned metanephric cells appeared to produce urine-like liquid whereas those without cells or seeded with allogeneic cells did not. Histological analysis of the explant revealed a well-developed renal structure comprised of organized glomeruli-like, tubular-like, and vascular elements that were clearly distinct from each other but continuous within the structure. These renal tissues therefore appeared integrally connected in a unidirectional manner to the reservoirs, resulting in the excretion of urine into the collecting system. Although it is not clear how the cultured cells digested from metanephros gained polarity and self-assembled into glomeruli and tubules, this technique successfully used nuclear transplantation for renal regeneration without the risks and long-term effects of immunosuppression.
Establishment of ‘self’ kidney from autologous mesenchymal stem cells.
summarizes the ideal features of an artificial kidney established de novo, based on these accumulating challenges. Due to the anatomical complexity of kidney and the need for residential cells to communicate with each other to produce urine, the artificial kidney structure must include glomerulus, tubules, interstitium, and vessels. It does not, however, need to be the same size as native kidney, as long as the glomerular filtration rate (GFR) exceeds 10 ml/min, although the volume must reach probably 10% of the volume of native kidney. Ideally, it should also be maintained and grow with no or minimum requirement of immunosuppression. In addition, an artificial kidney has to perform several important functions other than urine production, such as blood pressure control, calcium phosphorus balance, and erythropoietin (EPO) production.
With these features in mind, we attempted to establish an ideal artificial kidney, addressing the issues one by one. First, we aimed to reconstruct an organized and functional kidney structure using the developing heterozoic embryo as an “organ factory”. During embryogenesis, a single fertilized cell develops into a whole body within 266 days in human and 20 days in rodents. The neonate has every organ positioned correctly, indicating that a single fertilized ovum contains the information to build the body including kidney. We therefore sought to borrow this programming of a developing embryo by applying the stem cells at the time of organogenesis.
During development of the metanephros (the permanent kidney), the metanephric mesenchyme initially forms from the caudal portion of the nephrogenic cordCitation73 and secretes glial cell line-derived neurotrophic factor (GDNF), which induces the nearby Wolffian duct to produce a ureteric bud.Citation74 The metanephric mesenchyme consequently forms the glomerulus, proximal tubule, loop of Henle, and distal tubule, as well as the interstitium as a result of reciprocal epithelial-mesenchymal induction between the ureteric bud and metanephric mesenchyme.Citation75 For this epithelial-mesenchymal induction to occur, GDNF must interact with its receptor, c-ret, which is expressed in the Wolffian duct.Citation75 We hypothesized that GDNF-expressing mesenchymal stem cells may differentiate into kidney structure if positioned at the budding site and stimulated by numerous factors spatially and temporally identical to those found in the developmental milieu.
To address this hypothesis, human mesenchymal stem cells (hMSCs) were initially injected into the developing metanephros in vitro, although this was not sufficient to achieve kidney organogenesis or even integration of hMSCs into the developing rodent metanephros. No kidney structure was established nor were any kidney-specific genes expressed,Citation76 suggesting that the hMSCs must be placed before the metanephros begins to develop, in a specific, defined embryonic niche to allow for exposure to the repertoire of nephrogenic signals required to generate the organ. This can best be achieved by implanting hMSCs into the nephrogenic site of a developing embryo. However, once embryos are removed for cell implantation, they cannot be returned to the uterus for further development. Therefore, we established a culture system, in combination with a whole embryo culture system, followed by metanephric organ culturing. This “relay culture” may allow development of the metanephros from those structures present before budding until the occurrence of complete organogenesis ex utero. In this system, embryos were isolated from the mother before budding and grown in a culture bottle until the formation of a kidney rudiment, so that it could be further developed by organ culture in vitro.Citation76 Using this combination, kidney rudiments continued to grow in vitro, as assessed by the observation of fine tubulogenesis and ureteric bud branching, indicating that the metanephros can complete development ex utero even if the embryo is dissected prior to sprouting of the ureteric bud.
Based on this result, hMSCs were microinjected at the site of budding () and subjected to this relay culture. Before injection, these hMSCs were genetically engineered to express GDNF temporally using adenovirus and also labeled with LacZ gene and DiI. Soon after the injection embryos together with placenta were transferred to the incubator for whole embryos (). After the relay culture, X-gal-positive cells were scattered throughout the metanephric rudiment and these were morphologically identical to tubular epithelial cells, interstitial cells, and glomerular epithelial cells.Citation76 RT-PCR also revealed the expression of several podocyte- and tubule-specific genes.Citation76 These data demonstrated that using a xenobiotic developmental process for growing embryos allows endogenous hMSCs to undergo an epithelial conversion and be transformed into an orchestrated nephron consisting of glomerular epithelial cells (podocytes) and tubular epithelial cells that are linked together. hMSCs can also differentiate into renal stroma after renal development.
We then proceeded to the next issue for artificial kidney establishment de novo i.e., urine production (, line 2). This requires that the formed kidney have the vascular system of the recipient, therefore the primary system must be modified to allow for vascular integration from the recipient to form a functional nephron. We utilized the findings of Rogers et al. described above,Citation66 whereby the metanephros can grow and differentiate into a functional renal unit with integration of recipients vessels if it is implanted into omentum. To incorporate this modification into our relay culture system, we needed to know how early a stage of metanephros could develop in the omentum. We transplanted metanephroi from different embryonic stages into omentum and found after two weeks that only metanephros rat embryos older than E13.5 developed successfully. Therefore, the relay culture system was modified so that organ culture was terminated within 24 h, by which time metanephros was allowed to develop sufficiently and the kidney primordia could be transplanted into the omentum (termed “modified relay culture system”). As a result, hMSCs-derived “neo-kidney” was generated that was equivalent to a human nephron ( and B).Citation77
To examine the origin of the vasculature in the neo-kidney, we generated LacZ-transgenic ratsCitation78 as recipients so that donor- and recipient-derived tissues were distinguishable by X-gal assay. The utility of genetically marked transgenic (tg) rats in organogenesis have been previously confirmed, while GFP marker has the antigenecity in rat.Citation79 Then, the LacZ tg rat which expresses marker gene ubiquitously was chosen to demonstrate vascular origin in our experiment.Citation77 After the modified relay culture, several vessels from the omentum appeared to be integrated into the neo-kidney () and X-gal staining showed that most of the peritubular capillaries were LacZ-positive, suggesting that they were of recipient origin. Furthermore, electron microscopic analysis revealed red blood cells in the glomerular vasculature (). These data indicated that the vasculature of the neo-kidney in the omentum originated from the host and communicated with the host circulation, suggesting its viability to collect and filter the host blood to produce urine. To check this, neo-kidney was left for four weeks in the omentum to develop further. Surprisingly, the structure developed hydronephrosis, supporting the capability of urine production (because the ureter was buried under the fat of the omentum with no egress for the urine, resulting in the hydronephrosis). Analysis of the liquid from the expanded ureter showed a higher urea nitrogen and creatinine level compared with the recipient sera, and one comparable with native urine (840.3 ± 184.6 vs. 30.4 ± 10.8 and 10.1 ± 3.1 vs. 0.3 ± 0.2, respectively).Citation77 We therefore concluded that the neo-kidney developed in the omentum was capable of producing urine by filtration of the recipient's blood.
Finally, we addressed the final goal for ideal regenerative kidney (, line 3). Kidney plays an important local role in removing uremic toxins and excess fluid by producing urine and also contributes systemically to the maintenance of homeostasis through hematopoiesis, blood pressure control, and calcium/phosphorus balance. We therefore asked whether the neo-kidney produced by our system was biologically viable using mouse models of hereditary renal diseases, focusing on Fabry disease. Fabry disease is an X-linked lysosomal storage disease that is caused by a α-galactosidase A (α-gal A) enzyme deficiency. It leads to the abnormal accumulation of glycosphingolipid with terminal α-galactosyl residues (Gb3) in various organs including the kidney, causing CRF.Citation80 Renal involvement is characterized by Gb3 deposits mainly in podocytes and tubular epithelial cells, resulting in glomerulosclerosis, tubular atrophy, and interstitial fibrosis.Citation80 Fabry mice lacking α-gal A appear normal and there are no significant differences in renal histology by PAS, Masson, and Sudan IV staining compared to wild-type mice (our unpublished data). This is because the accumulation of abnormal lipid is very slow and the mice die before manifestation of renal failure. We therefore assessed viability of the regenerated metanephros by α-gal A activity and clearance of abnormal Gb3 accumulation in these mice. To do this, hMSCs were transfected with GDNF and injected into E9.5 Fabry mouse embryos, and then subjected to the relay culture to regenerate kidney. Compared to wild type, the basal level of α-gal A bioactivity in the metanephros from the Fabry mouse was quite low, whereas metanephroi regenerated with hMSCs expressed significantly more α-gal A.Citation76 Gb3 started to accumulate within the ureteric bud and S shape bodies in the metanephros of the Fabry animals and this accumulation was markedly cleared by replacement of the hMSCs-derived nephrons, which possessed α-gal A activity.Citation76 These data indicated that a regenerated neo-kidney is able to maintain the local environment. Furthermore, we confirmed that the neo-kidney could produce human proteins and participate in human homeostasis. For example, we examined the nucleotide sequences of 1α hydroxylase, PTH receptor-1, and erythropoietin from RNA extracted from the neo-kidney and identified human-specific products (our unpublished data), suggesting that the established organ was integrated properly into the host endocrine system. Taken together, these data suggest that the neo-kidney developed in the omentum may fulfill normal renal functions in addition to urine production.
As the next step, we sought to test larger host embryos to establish larger organs that are more suited for use in humans (, line 4). It was reported recently that pig metanephroi transplanted into rat omentum grew to a larger volume than a normal rat kidney.Citation81 We are therefore examining the applicability of pig for our system, although our understanding of renal development processes extends only to a few animal models.
In our experiments, we used primary hMSCs obtained from the bone marrow of healthy volunteers as a source of neo-kidney. hMSCs were shown recently to retain plasticity with the ability to differentiate into several different cells types, depending on their microenvironment.Citation82 Embryonic stem (ES) cells are another option as an origin of kidney regeneration.Citation83 Unlike hMSCs, ES cells that are injected into established metanephroi may be integrated into the renal structure during organ culturing,Citation84 suggesting that ES cells are feasible for forming renal structure compared with MSC. We previously found that hMSCs do not express WT1 and Pax2 (our unpublished data), suggesting that they do not manifest complete molecular features characteristic of the onset of normal nephrogenesis. In contrast to ES cells, however, adult MSCs can be isolated from autologous bone marrow and applied for therapeutic use without any serious ethical issues or the use of immunosuppressant (, line 5).
Perspectives and Conclusion
In this article, we have reviewed recent research in the field of regenerative medicine for kidney diseases and have proposed possible therapeutic application for acute and chronic renal failure in combination with the emerging knowledge of kidney stem cell biology and developmental biology. We know that prior to the total loss of renal structure, kidney function can be restored by reactivating quiescent renal stem cells and/or supplying renal stem cells expanded sufficiently in vitro. In contrast, if kidney structure is totally disrupted, which could happen in patients with end-stage renal damage undergoing long-term dialysis, the only cure might be the development of a functional whole kidney de novo.
Recent progress in stem cell biology has demonstrated that renal stem cells, with the capability to differentiate into mature renal cells, do exist in adult individual; however, the debate is ongoing regarding their major location. Suggestions include the interstitium of the cortexCitation24,Citation37 and papilla,Citation32 tubules,Citation27 and bone marrow.Citation18,Citation19 Recent evidence suggested that liver progenitor cells, called oval cells, which transdifferentiate into hepatocytes or biliary epithelial cells, may originate not only from the point where the terminal bile ducts meet the periportal hepatocytes, but also from the bone marrow.Citation85 It is equally likely that other stem cells, including those in the kidney, are not restricted to one place and may be supplied from different places depending on the severity, location, and duration of damage. However, resident tissue stem cells constitute only a small percentage of the total cellularity of an organ,Citation86 suggesting that tissue-specific stem cells are not sufficient in number for therapeutic regeneration after tissue injury and must either be expanded in vivo or supplied on demand from the circulation. Future studies need to provide a better understanding of what controls the contribution of renal stem cells in a given pathophysiological setting if they are to be applied therapeutically for human disease.
Establishment of functional whole kidneys de novo has not received much public attention due to the challenging and lengthy nature of advances in this field of research. This has changed recently with publication of the catastrophic costs for dialysis-related diseases. We need to be innovative in our therapy for CRF, and provide a substitute for dialysis as soon as possible. Regenerative medicine is the great hope for realizing this goal. Here, we have listed the minimum requirements for artificial kidney formation de novo and reviewed step by step the challenges in satisfying them. We have developed a culture system to allow embryonic metanephros to differentiate to complete organogenesis ex utero. We have also confirmed that hMSCs can differentiate and develop into part of the nephron in rodent embryos by following the program of kidney organogenesis. Such hMSCs-derived kidney anlagen may be developed further by combination with systems for “growing kidney in situCitation68”. With this modification, the hMSCs may successfully differentiate and grow into a functional neo-kidney, vascularized by in-growth of recipient blood vessels and able to produce urine. Unfortunately, the current form of neo-kidney is chimeric and derived from both hMCS and the host embryo tissues. We are currently attempting to overcome this issue by eliminating xenogenic cells from the graft before transplantation into the omentum using a transgenic host that carries a regulated suicide gene, and also by exchanging the posterior part of the Wolffian duct with a human form during its elongation so that the collecting system in the neo-kidney may be of host origin. Technical developments such as these may result in our approach leading to long-term renal replacement therapy in the future.
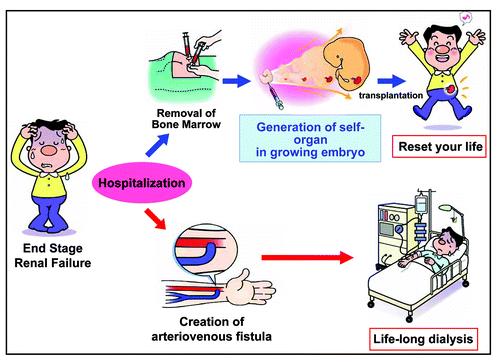
We would like to propose a putative scenario for the application of regenerative medicine to CRF therapy (). Bone marrow aspiration will be done from a patient with CRF instead of creating an A–V fistula and isolated MSCs will be cultured in growing embryos for a given time to develop into kidney, followed by autologous implantation into the omentum of the same patient. Kidney primordia with eventually become a ‘self’ organ that produces the patient's urine. The patient might enjoy relief from dialysis and completely reset his/her body without renal disease.
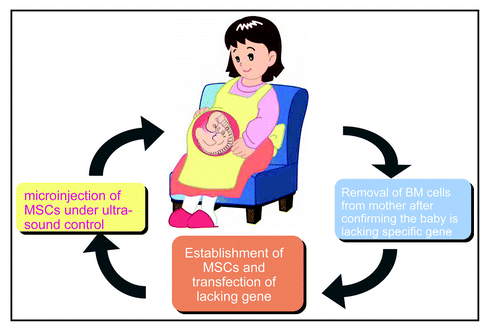
Kidney regeneration may also be applied for the treatment of hereditary renal diseases (). In this scenario, once a neonate is confirmed to lack a specific gene that causes an hereditary renal disease, such as Fabry disease, the bone marrow will be removed from the mother and mesenchymal stem cells will be established and transfected with the missing or defective gene. These cells will then be microinjected into the patient under ultrasound control. The new-born baby may then have the gene it would otherwise lack, and have it exclusively in the kidney.
It should be noted that regenerative medicine for renal diseases is still in the development phase and a long way from being established. It provides significant hope, however, for patients with renal disease who are dependent on dialysis for the rest of their lives. We believe an emerging knowledge of kidney stem cell biology and developmental biology will proceed to the next generation of therapeutic strategies for renal diseases as a regenerative therapy aimed at regaining damaged components or resetting the abolished function for the treatment.
Figures and Tables
Figure 1 Injection of human mesenchymal stem cells (hMSCs) into growing rat embryo at the sit of nephrogenesis. Using mouth pipette, hMSCs were injected into the intermediate mesoderm between the somite and the lateral plate at the level of somite 29, which we previously estimated by in situ hybridization for c-ret, to be the ureteric budding site. Asterisk indicates chorioallantoic placenta and arrow indicates yolk sac.
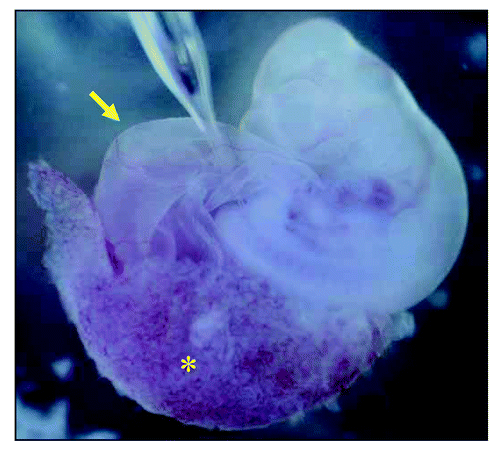
Figure 2 Appearance of the incubator for whole embryos. This system can rotate 15-ml culture bottles to achieve a continuous oxygen flow over the entire culturing period. The optimal gas-exchange schedules for rat embryo was determined previously.Citation76
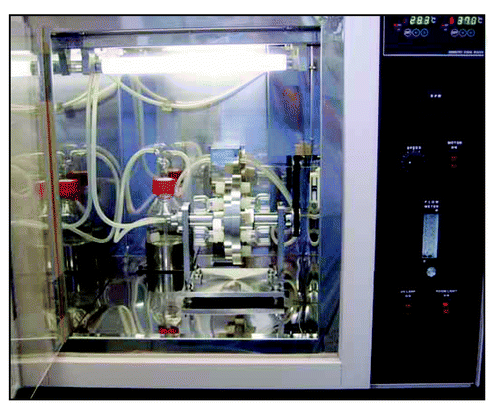
Figure 3 Generation of vascularized neo-kidney from hMSCs. LacZ-positive hMSCs were differentiated in vivo using the modified relay culture system (A). Yellow dotted lines indicate the outline of the developed kidney in the omentum (top). These were dissected out and compared with native kidney (bottom). The resulting neo-kidney was subjected to X-gal assay to trace the transplanted hMSCs (B, top). The morphology of these LacZ-positive cells (shown under high magnification, bottom) and the renal structures to which they contributed was consistent with their being glomerular cells (left) and tubular cells (right). High magnification imaging revealed that several vessels from the omentum were integrated into the no-kidney (C) and electron microscopic analysis showed that the glomerular vasculature contained red blood cells (arrows), which might have derived from the host circulation (D). Reproduced with permission.Citation77
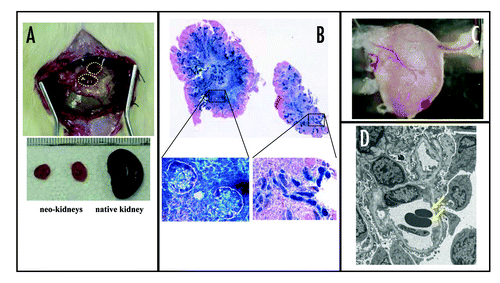
Table 1 List of minimum requirements for regenerated artificial kidney for chronic renal failure
Acknowledgements
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Naito Foundation. The transgenic rats are available from National Bio Resource for the Rat (Professor. Tadao Sarikawa, [email protected]) in Japan and Comparative Medicine Center and Research Animal Diagnostic Laboratory, College of Veterinary Medicine, University Missouri (Professor John Critser, [email protected]) in USA.
References
- Asahara T, Murohara T, Sullivian A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964 - 967
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: A hematopoietic fate adopted by adult neural stem cells in vivo. Science 1999; 283:534 - 537
- Thadhani R, Pascual M, Bonventre JV. Medical progress: Acute renal failure. N Engl J Med 1996; 334:1448 - 1460
- Schrier RW, Wang W, Poole B, Mitra A. Acure renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004; 114:5 - 14
- Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 2004; 351:159 - 169
- Krause D, Cantley LG. Bone marrow plasticity revisited: Protection or differentiation in the kidney tubule?. J Clin Invest 2005; 115:1705 - 1708
- Korbling M, Estrov Z. Adult stem cells for tissue repair-a new therapeutic concept?. N Engl J Med 2003; 349:570 - 582
- Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res 2002; 91:1092 - 1102
- Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol 2005; 90:315 - 326
- Kurokawa K, Nangaku M, Saito A, Inagi R, Miyata T. Current issues and future perspectives of chronic renal failure. J Am Soc Nephrol 2002; 13:S3 - S6
- Locatelli F, Vecchio LD, Possoni P, Monzoni C. Nephrology: Main advances in the last 40 years. J Nephrol 2006; 19:6 - 11
- Lysaght MJ. Maintenance dialysis population dynamics: Current trends and long-term implications. J Am Soc Nephrol 2002; 13:S37 - S40
- Paulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contribute to renal parenchymal turnover and regeneration. J Pathol 2001; 195:229 - 235
- Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int 2002; 62:1285 - 1290
- Imasawa T, Utsunomiya Y, Kawamura T, Zhong Y, Nagasawa T, Okabe M, Maruyama N, Hosoya T, Ohno T. The potential of bone marrow-derived cells to differentiate to glomerular mesangial cells. J Am Soc Nephrol 2001; 12:1401 - 1409
- Ito T, Suzuki A, Imai E, Okabe M, Hori M. Bone marrow is a reservoir repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol 2001; 12:2625 - 2635
- Poulsom R, Alison MR, Forbes SJ, Wright NA. Adult stem cell plasticity. J Pathol 2002; 197:441 - 456
- Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 2003; 14:1188 - 1199
- Kale SKA, Clark PR, Kashigarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 2003; 112:42 - 49
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 2005; 115:1743 - 1755
- Handgretinger R, Gordon PR, Leimig T, Chen X, Buhring HJ, Niethammer D, Kuci S. Biology and plasticity of CD133+ hematopoietic stem cells. Ann N Y Acad Sci 2003; 996:141 - 151
- Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res 2004; 95:343 - 353
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA 2000; 97:14720 - 14725
- Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol 2005; 166:545 - 555
- Challen GA, Martinez G, Davis MJ, Taylor DF, Crowe M, Teasdale RD, Grimmond SM, Little MH. Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol 2004; 15:2344 - 2357
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, ROmagnani R, ROmagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 2006; 17:2443 - 2456
- Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J 2005; 19:1789 - 1797
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implication for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990; 61:1329 - 1337
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 1999; 116:7 - 14
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenbironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001; 24:671 - 681
- Maeshima A, Yamashita S, Mojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 2003; 14:3138 - 3146
- Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest 2004; 114:795 - 804
- Googell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996; 183:1797 - 1806
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Kagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001; 7:1028 - 1034
- Jackson K, Mi T, Goodwell M. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 1999; 96:14482 - 14486
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999; 401:390 - 394
- Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K, Ichiyanagi T, Kohike H, Komori T, Takahashi I, Takase O, Imai N, Yoshikawa M, Inowa T, Hayashi M, Nakaki T, Nakauchi H, Okano H, Fujita T. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J Cell Biol 2005; 169:921 - 928
- Lu J, Webb R, Richardson JA, Olson EN. MyoR: A muscle-restricted basic helix-loop-helix transcription factor that antagonizes the action of MyoD. Proc Natl Acad Sci USA 1999; 96:552 - 667
- Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 2005; 115:1756 - 1765
- Kawaida K, Matsumoto K, Schimizu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA 1994; 91:4357 - 4361
- Miller SB, Martin DR, Kissane J, Hammerman MR. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am J Physiol 1994; 266:F129 - F134
- Bacallao R, Fine LG. Molecular events in the organization of renal tubular epithelium: From nephrogenesis to regeneration. Am J Physiol 1989; 257:F913 - F924
- Wallin A, Zhang G, Jones TW, Jaken S, Stevens JL. Studies on proliferation and vimentin expression after 32S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest 1992; 66:474 - 484
- Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moodby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol 1995; 128:171 - 184
- Sanson OS, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev Biol 1993; 160:293 - 302
- Karp SL, Oritiz-Arduan A, Li S, Nielson EG. Epithelial differentiation of metanephric mesenchymal cells after stimulation with hepatocyte growth factor or embryonic spinal cord. Proc Natl Acad Sci USA 1994; 91:5286 - 5290
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2 deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993; 75:229 - 240
- Knudon CM, Tung KSK, Tourtelotte WG, Brown GAJ, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995; 270:96 - 99
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development 1995; 121:4057 - 4065
- Basile DP, Liapis H, Hammerman MR. Expression of bcl-2 and bax in regeneration rat renal tubules following ischemic injury. Am J Physiol 1997; 272:F640 - F647
- Imgrund M, Grone E, Hermann-Josef G, Kretzler M, Holzman L, Schlondorff D, Rothenpieler UW. Re-expression of the developmental gene Pax2 during experimental acute tubular necrosis in mice. Kidney Int 1999; 56:1423 - 1431
- Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: Cellular and molecular regulation. Mech Dev 2000; 92:31 - 45
- Nigam SK, Lieberthal W. Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol 2000; 279:F3 - F11
- Yoshino J, Monkawa T, Tsuji M, Hayashi M, Saruta T. Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol 2003; 14:3090 - 3101
- Davies JA, Bard JBL. The development of the kidney. Curr Top Dev Biol 1998; 39:245 - 301
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-b1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003; 9:964 - 968
- Maeshima A, Zhang YQ, Nojima Y, Naruse T, Kojima I. Involvement of the activin-follistatin system in tubular regeneration after renal ischemia in rats. J Am Soc Nephrol 2001; 12:1685 - 1695
- Maeshima A, Maeshima K, Nojima Y, Kojima I. Involvement of Pax-2 in the action of activin A on tubular cell regeneration. J Am Soc Nephrol 2002; 13:2850 - 2859
- Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 2001; 128:3105 - 3115
- Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development 2005; 133:151 - 161
- Araki T, Hayashi M, Saruta T. Cloning and characterization of a novel gene promoting ureteric bud branching in the metanephros. Kidney Int 2003; 64:1968 - 1977
- Yokoo T, Ohashi T, Utsunomiya Y, Shen JS, Hisada Y, Eto Y, Kawamura T, Hosoya T. Genetically modified bone marrow continuously supplies anti-inflammatory cells and suppresses renal injury in mouse Goodpasture syndrome. Blood 2001; 98:57 - 64
- Broxmeyer HE, Gluckman E, Auerbach A, Douglas GW, Friedman H, Cooper S, Hangoc G, Kurtzberg J, Bard J, Boyse EA. Human umbilical cord blood: A clinically useful source of transplantable hematopoietic stem/progenitor cells. Int J Cell Cloning 1990; 8:76 - 91
- Yokoo T, Ohashi T, Utsunomiya Y, Okamoto A, Suzuki T, Shen JS, Tanaka T, Kawamura T, Hosoya T. Gene delivery using human cord blood-derived CD34+ cells into inflamed glomeruli in NOD/SCID mice. Kidney Int 2003; 64:102 - 109
- Woolf AS, Palmer SJ, Snow ML, Fine LG. Creation of functioning chimeric mammalian kidney 1990; 38:991 - 997
- Rogers S, Lowell JA, Hammerman NA, Hammerman MR. Transplantation of developing metanephroi into adult rats. Kidney Int 1998; 54:27 - 37
- Dekel B, Marcus H, Herzel BH, Bucher WO, Passwell J, Yair R. In vivo modulation of the alogeneic immune response by human fetal kidneys: The role of cytokines, chemokines and cytolytic effecter molecules. Transplantation 2000; 69:1470 - 1478
- Hammerman MR. Tissue engineering the kidney. Kidney Int 2003; 63:1195 - 1204
- Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH, Reisner Y. Human and porcine early kidney precursors as a new source for transplantation. Nat Med 2002; 9:53 - 60
- Chan T, Ariizumi T, Asashima M. A model system for organ engineering: Transplantation of in vitro induced embryonic kidney. Naturwissenschaften 1999; 86:224 - 227
- Okabayashi K, Asashima M. Tissue generation from amphibian animal caps. Curr Opin Genet Dev 2003; 13:502 - 507
- Lanza RP, Chuug HY, Yoo JJ, Wettestein PJ, Blackwell C, Borson N, Hofmeister E, Schuch G, Soker S, Moraes CT, West MD, Atala A. Generation of histocompatible tissues using nuclear transplantation. Nat Biotech 2002; 20:689 - 696
- Saxen L. Organogenesis of the kidney 1987; Cambridge, UK Cambridge University Press
- Davies JA, Fisher CE. Genes and protein in renal development. Exp Nephrol 2002; 10:102 - 113
- Lipschuts JH. Molecular development of the kidney: A review of the results of gene disruption studies. Am J Kid Dis 1998; 31:383 - 397
- Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, Takahashi M, Terada Y, Eto Y, Kawamura T, Osumi N, Hosoya T. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissue. Proc Natl Acad Sci USA 2005; 102:3296 - 3300
- Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, Kawamura T, Hosoya T, Okabe M, Kobayashi E. Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol 2006; 17:1026 - 1034
- Inoue H, Osawa I, Murakami T, Kimura A, Hakamata Y, Sato Y, Kaneko T, Okada T, Ozawa K, Francis J, Lione P, Kobayashi E. Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem Biophys Res Commun 2005; 329:288 - 295
- Sawada H, Sheng HM, Hakamata Y, Esaki M, Kita A, Yoshida T, Kobayashi E. Contribution of subcutaneous connective tissues to the epithelialization and cyst formation by the skin transplanted subcutaneously. Organogenesis 2004; 1:55 - 59
- Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol 2002; 13:S134 - S138
- Hammerman MR. Renal organogenesis from transplanted metanephric primordia. J Am Soc Nephrol 2004; 15:1126 - 1132
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276:71 - 74
- Yamamoto M, Cui L, Johkura K, Asanuma K, Okouchi Y, Ogiwara N, Sasaki K. Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Renal Physiol 2005; 290:F52 - F60
- Steenhard BM, Isom KS, Cazcarro P, Dunmore JH, Godwin AR, St John PL, Abrahamson DA. Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol 2005; 16:1623 - 1631
- Fobes SJ, Poulsom R, Wright NA. Hepatic and renal differentiation from blood-borne stem cells. Gene Ther 2002; 9:625 - 630
- Alison MR, Poulsom R, Forbes S, Wright NA. An introduction to stem cells. J Pathol 2002; 197:419 - 423