Abstract
A non-eukaryotic, metakaryotic cell with large, open mouthed, bell shaped nuclei represents an important stem cell lineage in fetal/juvenile organogenesis in humans and rodents. Each human bell shaped nucleus contains the diploid human DNA genome as tested by quantitative Feulgen DNA cytometry and fluorescent in situ hybridization with human pan-telomeric, pan-centromeric and chromosome specific probes. From weeks ~5-12 of human gestation the bell shaped nuclei are found in organ anlagen enclosed in sarcomeric tubular syncytia. Within syncytia bell shaped nuclear number increases binomially up to 16 or 32 nuclei; clusters of syncytia are regularly dispersed in organ anlagen. Syncytial bell shaped nuclei demonstrate two forms of symmetrical amitoses, facing or “kissing“ bells and "stacking" bells resembling separation of two paper cups. Remarkably, DNA increase and nuclear fission occur coordinately. Importantly, syncytial bell shaped nuclei undergo asymmetrical amitoses creating organ specific ensembles of up to eight distinct closed nuclear forms, a characteristic required of a stem cell lineage. Closed nuclei emerging from bell shaped nuclei are eukaryotic as demonstrated by their subsequent increases by extra-syncytial mitoses populating the parenchyma of growing anlagen. From 9–14 weeks syncytia fragment forming single cells with bell shaped nuclei that continue to display both symmetrical and asymmetrical amitoses. These forms persist in the juvenile period and are specifically observed in bases of colonic crypts. Metakaryotic forms are found in organogenesis of humans, rats, mice and the plant Arabidopsis indicating an evolutionary origin prior to the divergence of plants and animals.
Introduction
Observations in human hindgut at the ∼7th week of gestation discovered peculiar bell shaped nuclei ensheathed in tubular syncytia that underwent both symmetrical “stacked cup” amitoses and asymmetrical amitotic fissions in which any of some eight diverse nuclear forms were observed to emanate from bell mouths within the syncytia.Citation1 Single cells containing each of the various forms of closed nuclei arising from asymmetric amitoses were found to increase by mitoses that created and preserved the diverse closed nuclear forms and populated the growing tissue. The reason that these nuclear forms lay undiscovered appeared to be their marked lability. The bell morphology was found to degenerate into a globular mass by 30–45 minutes after surgical removal even when held in cold cell culture media.Citation1
The qualities of growth by symmetric nuclear fissions and creation of differentiated cell forms by asymmetrical fissions were recognized as characteristics expected of a fetal organogenic stem cell lineage.Citation1 The modes of amitotic nuclear fission distinguished the open bell shaped nuclei from closed mitotic eukaryotic nuclei and they were denominated “metakaryotic.” Metakaryotic cells displaying bell shaped nuclei with similar amitotic fissions were observed in the crypt bases of colonic pre-neoplastic adenomas, adenocarcinomas and their liver metastases suggesting they also served as a carcinogenic stem cell lineage. In particular, colonic adenomas displayed microcolonies with 4, 8, …128 cells consisting of one cell with a bell shaped nucleus and 2n-1 cells with identical closed nuclear morphotypes that populated adenomatous crypts and aberrant crypt-like structures. Normal adult colonic crypt bases rarely (∼0.1%) displayed a bell shaped nucleus.
However, while the original observations drew from multiple examples of colonic adenomas and adenocarcinomas, observations during human fetal development were limited to the hindgut of a single fetus, clearly insufficient to support the hypothesis put forward that metakaryotic cells could serve as a general form of stem cell lineage among organs in humans and other species. Herein we apply a modified approach to preparation of tissue samples within fifteen minutes of surgical removal in which tissue “maceration” 1 by 45% acetic acid has been replaced by partial digestion with collagenase to a broad spectrum of tissues from humans and rodents.
In our observations we addressed several important questions that arose in response to the original report. Were these structures some form of parasite, i.e., did they contain the human DNA complement? Were they limited to the colon? When did they appear and disappear in organ development? Were the “tubular syncytia”Citation1 related to the syncytial “primary myotubes” that have similar size and nuclear number but have been considered as a specialized stage of muscle development (myogenesis)? Are these nuclear forms found in development of other species?
Herein these questions are addressed. Observations are reported from various organs of more than a hundred fetuses ranging from ∼5 to 16 weeks of gestation and multiple samples from normal adult colon. Similar observations are reported from fetal mice and rats, a mouse and human cell culture and the stem emerging from the germinated seed of the plant Arabidopsis.
Results
Observations have been organized in order of increasing scale: images of bell shaped nuclei in amitoses (), in nuclei of sarcoplasmic syncytia (), of syncytia in clusters (), of post-syncytial bell shaped nuclei dispersed in meta-organs (). Metakaryotic nuclei and syncytia from rodents and Arabidopsis are shown in .
From the first recognition of fetal anlagen (5 wk) to the assumption of the general form of partially developed metaorgans (∼12 wk) large, open mouthed, bell shaped nuclei were primarily found ensheathed in tubular syncytia with sarcomeric striations in the syncytial wall in all tissues examined: colon, small intestine, stomach, esophagus, urinary bladder, heart muscle, skeletal muscle, bone, spinal cord and brain. In this the original observations in a single fetal hindgutCitation1 were confirmed and extended to these other organs.
Two forms of amitosis concordant with DNA increase.
Bell shaped nuclei were observed in all organs studied as single nuclei or involved in one of two forms of symmetrical amitoses (). In the earliest gestational ages examined, 5–7 weeks, a few bell shaped nuclei appeared as “kissing” bell amitotic fission structures or as single cells without any sign of syncytial sarcomeric structures. Soon thereafter bell shaped nuclei were found exclusively within syncytia in which “stacking” bell symmetrical amitotic fissions or single bell shaped nuclei were observed (). When kissing bell fission structures were found in syncytia they were generally, but not always, found at the midpoint of the syncytium.
Quantitative DNA imaging cytometry determined that the DNA in each separate bell shaped nucleus was the same as found in diploid human lymphoblastoid cells used as internal standards and one half the DNA found in extra-syncytial anaphase-telophase fetal nuclei in the same slide preparation. These measurements indicated that the bell shaped nuclei contained a normal human diploid amount of DNA (∼6 pkg). This observation was confirmed and extended with fluorescent human specific probes for centromeric and telomeric sequences as well as for two specific human chromosomes (#18 and #6) that stained all bell shaped nuclei and closed nuclear forms derived from bell shaped nuclei by asymmetrical amitotic fissions (manuscript in preparation).
Condensed DNA forming two concentric rings at the mouth of each bell shaped nucleus ( and F) was found to comprise some 10% of the total nuclear DNA. DNA increase was first seen as a doubling of the two pairs of concentric condensed DNA rings ( and H) increasing the total amount of DNA to 110% of the interphase DNA content. Other images () revealed increasing amounts of DNA as the two pairs of condensed rings physically separated. When bell shaped nuclei separated a total DNA of twice that in a single nucleus was observed ( and K). As shown in , a series of “stacking” and “kissing” amitotic figures drawn from human gut, spinal cord and brain 5–9 wks gestation, when arranged in order of separation of concentric rings created a monotonic series of increasing DNA content from about 6 to 12 pkg DNA. It thus appears that in both forms of amitosis, “kissing” and “stacking” bells, DNA segregation precedes or occurs at or about the same time as DNA synthesis.
These observations indicate amitotic processes of DNA synthesis and segregation distinct from eukaryotic cell forms in which the DNA synthetic period, S-phase, is separated by several hours from genomic segregation, mitosis, in embryonic and parenchymal cell of tissues in humans and other species. Cells or syncytia with bell shaped nuclei displaying both symmetric and asymmetric amitoses in meta-organs and tumors were dubbed “metakaryotic”Citation1,Citation2 on the basis of their distinctive cytomorphology. The present observations add concomitant DNA segregation and synthesis during amitotic nuclear fission as a metakaryotic quality.
Images of either symmetrical form of amitosis could be formally interpreted as either fission or fusion in which DNA is either increased or destroyed during the respective possibilities. However, the rapid monotonic, approximately exponential, increase in bell shaped nuclei in syncytia of organ anlagen with gestational age supports the interpretation of nuclear fission with concomitant net DNA increase.
Syncytial organization.
The tissues studied at the earliest gestational age available (∼5–7 wk) demonstrated single syncytia with but 4–16 bell shaped nuclei, numerous amitotic fission figures and sarcomeric striations on the syncytial wall ( and B). Bell shaped nuclei apparently increased synchronously by binomial fission within syncytia because nearly all syncytia contained 4, 8, 16 or 32 nuclei. Syncytia with at least one amitotic figure generally contained multiple amitotic figures consistent with synchronization of a nuclear division cycle within syncytia ( and C). Study of multiple meta-organs of more than one hundred fetuses of the first trimester always found syncytia with bell shaped nuclei. But in only ∼10% of fetuses were syncytia with amitotic figures found. In such cases amitotic figures were found within multiple meta-organs of the particular fetus. This distribution of syncytial amitotic figures within and among different fetuses combined with the seemingly synchronized syncytial amitotic divisions suggested a diurnal rhythm or other Zeitgeber synchronizing amitoses within each fetus.
The process by which syncytia themselves increase in number is not clear. Single bell shaped nuclei are observed in a position to emerge from the ends of tubular syncytia and may serve as origins of new syncytia (). However, each syncytium could have arisen from a precursor mitotic embryonic stem cell. By whatever processes, it is clear that the number of syncytia and the number of bell shaped nuclei per syncytium increased with gestational age in all fetal anlagen examined.
As reported for the fetal colon,Citation1 the fetal anlagen (5–12 weeks) and meta-organs (>12 weeks) of all tissues examined were populated with cells displaying closed mitotic nuclear forms ranging in size and shape from the ∼6 micron condensed spherical form through the 20 micron cigar—to the ∼40 micron sausage-shaped nuclei. Each of several distinct forms of closed nuclei appeared to emerge from the mouth of a bell shaped nucleus and subsequently give rise to an extra-syncytial colony of these nuclear isoforms by mitosis as originally reported.Citation1 Each syncytium appears to synchronously create a set of identical closed nuclei, i.e., a syncytium has not yet been found with more than one form of closed mitotic nucleus (). Quantitative Feulgen cytometry finds the same total human diploid DNA content in each of the closed nuclear isoforms. While the eight specific nuclear morphotypes previously reported in fetal gut were found among the various tissues, observations made to date are not yet sufficient to indicate specific numerical distributions of each form in each tissue anlagen. As syncytia proliferated increasing fractions demonstrated asymmetrical amitotic fissions. By week 12 symmetrical amitoses had become a small fraction of all syncytial amitoses as asymmetrical amitoses predominated numerically.
Syncytial clustering.
Syncytia with bell shaped nuclei demonstrating amitoses were ordered as structures parallel with nascent myofibers in cardiac and skeletal muscle (). In all other tissues syncytia appeared as clusters of ≤32 syncytia () and large super-clusters (). However, the mode of tissue maceration and gentle spreading used for these observations must have disturbed the three dimensional relationships of syncytia in clusters and super clusters as syncytia are very large, some 200–300 microns in length. Syncytial clusters and super-clusters were observed with very little variation in nuclear numbers among syncytia suggesting a synchronous symmetric division process operating among syncytia. Examination of the same organ anlagen in multiple fetuses leaves the impression that the super-clusters are regularly dispersed in time and space, a contention that will require a complete three dimensional quantitative imaging expedition to test. As originally reported,Citation1 syncytia display a noticeable autofluorescence in Carnoy fixed specimens. However, this autofluorescence was significantly less intense than the fluorescence observed in syncytia after staining with Feulgen reagent.
Post-syncytial fetal/juvenile phenomena.
Syncytial narrowing and fragmentation are observed as early as week 9 and as late as week 14. Images from the brain in week 13 are shown in . By 14 weeks of gestation the syncytia have disappeared. However, single cells with bell shaped nuclei equal or greater in number than those observed earlier in gestation in anlagen syncytia are regularly dispersed throughout the metaorgans as shown for the small intestine in .
The post-syncytial bell shaped nuclei are all physically associated with large Feulgen fluorescent, balloon shaped structures devoid of DNA (). These structures are presumably analogous to the cytoplasm of eukaryotic cells. They stain positive for mucus; acridine orange staining generally but not always reveals large amounts of red-orange fluorescence associated with rRNA. Note should be made that the autofluorescence observed in Carnoy fixed syncytial specimens was not observed in Carnoy fixed extra-syncytial metakaryotic cells. However, after Feulgen staining for DNA, balloon shaped cytoplasms in post-syncytial metakaryotes displayed intense fluorescence. The fluorescence is so intense that it suggests a Schiff's base reaction has taken place with an aldehydic moiety of fetal mucus yielding a previously unreported fluorescent product. This unknown reaction product provides a simple way to detect and enumerate such free metakaryotic cells in fetal/juvenile tissues and derived tumors ().
Asymmetrical amitoses continue in the post-syncytial period as may be seen by observing nuclei of the closed mitotic form emerging from bell shaped nuclei into the cytoplasmic structures (). Observations in later gestation, neonatal and pediatric surgical discard specimens are in progress; preliminary observations have found many single metakaryotic cells suggesting persistence in human juvenile tissues at higher levels than reported for adult colon.Citation1
Metakaryotic phenomena in rats, mice and a plant.
Bell shaped nuclei are found in forms remarkably similar to those found in humans in the fetuses of mice and rats and also in the emerging stems of the plant Arabidopsis (). Syncytia with “stacking” bell amitoses are clearly evident in rodent anlagen (). Syncytial behavior including symmetrical and asymmetrical amitoses () in mice and rats are similar to humans. Phase contrast images of unfixed cells from these rodent fetuses () captured bell shaped nuclei in asymmetrical amitoses indicating that these phenomena are not created by fixation and cell spreading procedures. Bell shaped nuclei were similarly observed at a frequency of about 1/10,000 in a mouse fetal myo- fibroblast line created and provided by Dr. James Sherley, Boston Biological Research Institute, Watertown, MA. Cell cultures were simply fixed with Carnoy's fixative without collagenase treatment or spreading on microscope slides.
Discussion
Are bell shaped nuclei artifacts?
Reasonable scientists have demanded a fuller explanation of how putatively important cell types with such marked size and morphological features evaded detection by 150 years of modern histology and pathology. Why weren't they observed and reported by the generations of microscopists dating back to Leeuwenhoek and Hooke? Two technical reasons can be discerned:
Bell shaped nuclei collapse into amorphous DNA masses 30–45 minutes after surgical removal from the body in fetal and tumor tissue of the colon.Citation1 Much histological detail is preserved in tissue held on ice or in cold physiological saline for hours post surgery but not the shape of bell shaped nuclei.
Bell shaped nuclei and syncytia are much larger than the 5-micron sections typically prepared for clinical pathologists' review. Uncut bell shaped nuclei are easily visualized by phase contrast microscopy in 30 µm flash frozen sections (). However, only a few nuclei are oriented to reveal their bell shape in thin cross sections as in standard H&E pathology preparations. They can, however, be found in standard H&E thin sections of tumors if one is searching for the distinctive bell shape (Dr. Lawrence Zukerberg, Pathology Unit, Massachusetts General Hospital, Boston, MA, USA, personal communication).
Some reviewers have wondered if the bell shaped nuclei could be artifacts of Feulgen staining procedures, maceration and spreading and/or Carnoy fixation. To address the possibility of staining artifacts images of identical forms of bell shaped nuclei from several tissue, tumor and cell culture sources are shown here as unstained phase contrast images () or stained with DAPI ( and F), hematoxylin-eosin (H&E) ( and H) acridine orange (), or with Feulgen reagent ( and L). Regarding fixation artifacts shows one of many bell shaped nuclei observed directly in flash frozen specimens. To respond to the possibility that maceration and spreading of tissues on microscope slides has created the bell shaped nuclear forms images of are offered of bell shaped nuclei in mouse fetal myocyte () and human adenoma derived () cell lines that have been neither macerated nor spread, simply fixed on their growth surfaces. These images obtained from multiple tissues, tumors, species and cell cultures and attested by the authors from four separate research centers appear to represent a previously unrecognized nuclear form that, based on images indicating both symmetrical and asymmetrical amitotic nuclear fissions, deserves careful study as a putative developmental lineage linking the mitotic embryonic stem cells and the mitotic parenchymal cells that populate fetal, juvenile and adult organs.
Amitosis in development of higher life forms.
Amitoses as a part of organogenesis has had a varied cytological history with relatively few researchers offering evidence of exceptions to the numerically more frequent and more easily recognized mitoses.Citation3–Citation5 However, the observations that preceded this reportCitation1 and the images provided herein appear to be the first to fulfill the stringent requirements put forward by Theodor BoveriCitation6 challenging a proponent of amitotic cell division in organogenesis to demonstrate that products of amitotic fission (a) gave rise to separate cells with separate nuclei and cytoplasm that (b) said separate nuclei subsequently divided by mitosis with the correct number of chromosomes.
The literature of histopathology also records many images of single cells and syncytia with the general morphological properties associated here with metakaryotic cells simply without recognition of their involvement in symmetrical and asymmetrical amitoses and thus without recognition of potential stem cell-like functions. For instance, “goblet cells” and “signet ring cells” reported variously in developing human organs,Citation7 ex vivo fetal outgrowths,Citation8 healing woundsCitation9 and carcinoid areas of tumorsCitation10 are variously described as having large, mucus-filled, balloon shaped cytoplasms with eccentric “thecal” or “horn shaped” nuclei. Goblet cell increases in healing wounds or fetal tissue explants have been interpreted as the creation of terminally differentiated post-mitotic cells because no mitoses of the eccentric nuclei have been observed.Citation7 It is thus possible that all or a subset of said goblet and/or signet ring cells have bell shaped nuclei that increase by amitoses. At least one report specifically hypothesized that cells with large, balloon shaped cytoplasms and eccentric nuclei found in a human juvenile brain were stem cells.Citation11
As shown herein a wide variety of developing tissues contain bell shaped nuclei undergoing syncytial and post syncytial symmetric and asymmetric amitoses. These syncytial structures are clearly not limited to developing skeletal and cardiac muscle. They have been found in all fetal organ anlagen examined to date, colon, small intestine, stomach, esophagus, urinary bladder, cardiac muscle, skeletal muscle, bone, spinal cord and brain representing tissues derived from each of the three primordial germ layers.
A restricted question: are tubular syncytia the myotubes of myogenesis?
Sarcomeric tubular syncytia with ∼16–32 nuclei several hundred microns in length, have been extensively reported as “primary myotubes” in skeletal and cardiac muscle development. These have been observed to originate in the earliest skeletal muscle anlagen of the 5th week of gestation and to persist through the 12th week when they give way to highly multinucleated skeletal muscle fibers and “satellite cells”. Primary myotubes have thus been clearly recognized as part of a stem cell lineage but have been interpreted to be elements specific to myogenesis alone. The nuclei of the primary myotubes were hypothesized to arise from fusion of extra-syncytial nuclei with the syncytium as no mitoses were observed during enlargement of “primary myotubes.”Citation12
However, clearer images reported here of sarcomeric tubular syncytia containing bell shaped nuclei undergoing symmetrical amitoses have the size, nuclear number and position in developing cardiac () and skeletal ( and C) muscle reported for primary myotubes. The longstanding question of whether primary myotube nuclei formed by amitoses or fusion with extra-syncytial cells should be once again examined incorporating these amitotic fission figures with in the tubular syncytia (myotubes) of developing muscle. Elegant molecular studies offered in support of the fusion hypothesis showed that nuclei from both contributors of a mouse chimera were located in myofibers of adult skeletal muscle but not cardiac muscle.Citation13 However, myofibers can be several centimeters in length containing thousands of nuclei, far more than the ∼32 of primary myotubes or tubular syncytia. A unifying hypothesis would envision formation of tubular syncytia/primary myotubes by amitotic monoclonal expansion to 32 nuclei followed by fusion of myotubes to create multiclonal skeletal myofibers. Preliminary observations have revealed what appears to be such end-on fusion.
The possibility that metakaryotes are restricted to a smooth muscle cell lineage.
Nearly all organs contain smooth muscle cells especially in vascular walls. The tubular syncytia and succeeding free cells with bell shaped nuclei found throughout organ anlagen might have been a stem cell lineage restricted solely to the smooth muscle cells. This interpretation was, however, discordant with images of asymmetric amitoses of syncytial and post syncytial bell shaped nuclei giving rise to cells with a wide and diverse set of multiple closed nuclear forms as, for instance, in . Syncytial and mononuclear bell shaped nuclei undergo multiple forms of asymmetric amitoses giving rise to a menagerie of closed nuclear forms that subsequently increase in number by mitoses and populate the growing anlagen and meta-organs ( and ). These closed nuclear forms include condensed shapes approximating spheres (∼6 µm), spheres (∼9 µm), ovals (∼10 µm), beans (∼15 µm), bullets (∼15 µm), cigars (15–20 µm), spindles (brain only, 15–30 µm) and sausages (∼40 µm).Citation1 Within a syncytium, the closed nuclear forms appear between bell shaped nuclei; each syncytium contains but one form of closed nucleus as in . All closed nuclear forms have been observed to undergo extra-syncytial mitoses in which the nuclear form is preserved through prophase with elongated but tightly paired chromatids ().Citation1 Together these images suggest that while some syncytia with bell shaped nuclei may lead to smooth muscle cells with small elliptical nuclei others lead to tissue parenchymal cell types with spherical and other nuclear forms. A key observation supporting this inference is the presence of single bell shaped nuclei specifically located at the bases of crypts of colonic adenoma, adenocarcinomas and their derived metastases.Citation1 Together these observations provide evidence that cells with bell shaped nuclei represent an important, but not necessarily the sole, stem cell lineage of organ growth and development.
Unknown role of metakaryotic cells as adult stem cells in wound healing.
The stem cell lineage leading to adult maintenance stem cells responsible for steady state turnover of tissues may be distinct from a lineage that lies dormant in readiness to respond to tissue damage.Citation2 Either or both could be derived from fetal/juvenile metakaryotic stem cells. As Cohnheim postulated in the 19th century mitotic, embryonic/fetal (stem) cells may persist in adults.Citation14,Citation15 Given the fact that metakaryotic phenomena were not recognized until recently, there might well be other stem cell lineages that have not yet been recognized.
Evolutionary origins of eukaryotes and metakaryotes.
As these metakaryotic phenomena are common to multiple differentiated tissues in humans and rodents ( and E–I) and bell shaped nuclei have also been found during development of the plant Arabidopsis () it appears that this form of amitotic nuclear fission is employed in the processes of post-embryonic differentiation in Metazoans generally if not universally. It is possible that some Metazoans might differentiate fully within the bounds of an embryonic/mitotic/eukaryotic developmental cascade without use of the post-embryonic/amitotic metakaryotic intermediary cascade. The metakaryotic cellular form, like the mitochondrion, probably had an evolutionary form independent of the mitotic forms of life and joined at some point prior to separation of plants and animals.
Indication of DNA segregation preceding DNA doubling.
The images of the two forms of symmetric amitoses of bell shaped nuclei can be logically arranged in order of increasing degree of separation to suggest that the processes of DNA synthesis and segregation are taking place in the same time period (). Time-lapse images of metakaryotic growth ex vivo should soon be available. In a live human adencarcinoma cell line using phase contrast optics, one of us (WGT) has observed a “kissing bell” form of amitotic fission taking place over a period of less than thirty minutes with complete separation of the daughter cells that maintained their nuclear bell shapes. The processes by which DNA segregation and synthesis can occur more or less simultaneously during amitoses in bell shaped nuclei are at this point enigmatic but nonetheless important. Research focusing on these phenomena is in progress.
Mutator/hypermutable phenotype in metakaryotic stem cell lineage.
Some indications as to other biological properties of the metakaryotic stem lineage are emerging. Studies of nuclear and mitochondrial mutant cluster size and number in human lungs has led to the conclusion that the late fetal/juvenile period is a time of high and constant mutation rates per fetal/juvenile stem cell doubling but tissue mutant fractions further increased by age or even smoking habits in adults.Citation16–Citation18 These observations in human lungs are concordant with the finding that mutant colony number is increased by solar irradiation in juvenile but not adult skin.Citation19,Citation20 Insofar as it may be logically argued that stable mutant clusters in adult tissues must have arisen by mutation in the stem cell lineage of the organ, the concomitant mode of DNA synthesis and segregation in the metakaryotic stem cell lineage may be logically associated with a mutator/hypermutable phenotype and thus to the initiating genetic changes of carcinogenesis.Citation2,Citation17 (Germ cell lineages have very low mutation rates per doubling and are thus probably not using metakaryotic intermediates with mutator properties).Citation21 These distinctly non-eukaryotic forms of life were in important ways anticipated by the observations and deductions of Okuyama and MishinaCitation22,Citation23 who hypothesized that cancer cell types included atavistic, even amitotic, forms specifically adapted to survival on a planet heretofore less hospitable to life.
Methods
Acquisition and fixation of tissue samples.
All samples were obtained under a protocol approved in advance by the MIT Committee on the Use of Humans as Experimental Subjects and the IRBs of each contributing hospital. Anonymous surgical discards were fixed immediately upon surgical removal in Carnoy's solution as describedCitation1 and stored under refrigeration in 70% ethanol.
Tissue preparation feulgen staining.
The use of 100 micron sections, tissue maceration and spreading, Feulgen staining and quantitative densitometry including image processing equipment have been reported as a step-by-step protocol.Citation1 An important variation introduced here is the use of a 1-hour incubation of fetal tissue sections with collagenase II (15 U/µL), 37°C, followed by spreading in a drop of 45% acetic acid to achieve milder conditions of maceration. N.B., 30 micron frozen sections permit direct visualization of the various forms of nuclei described without maceration and spreading indicating the forms are not procedural artifacts. The requirement for fixation or fast freezing of tissues within thirty minutes, preferably fifteen, of surgical removal appears to be very important. Images of bell shaped nuclei from colon anlagen and tumors are indistinguishable from lumpy spherical nuclei forty-five minutes after surgical removal.Citation1
Fluorescence in situ hybridization.
FISH was accomplished by using a combination of standard procedures with some modifications of the recommended protocols for a pan-telomeric PNA probe with a pan-centromeric and chromosome (#18) DNA probes.Citation22 Briefly, slides were pretreated with RNAse, pepsin, magnesium chloride and formaldehyde. They were hybridized with a PNA telomeric probe Cy-3-(CCCTAA)3, synthesized by Perseptive Biosystems, Framingham (MA, USA) followed by simultaneous hybridization with FITC-labelled pan-centromeric DNA probes (Cambio, UK). For detecting whole chromosome-specific DNA probes, FITC-labeled STAR*FISH for chromosome 18 was employed. The denaturation, hybridization, washing and mounting of the slides was performed exactly as described earlier.Citation24
Summary
The photo micrographic images offered herein demonstrate that metakaryotic phenomenon such as symmetrical and asymmetrical amitoses involving bell shaped nuclei are general in many developing human tissues and those of other Metazoan species including plants. More samples from human neonatal and juvenile tissues are being collected and the earliest stages of gestation are being explored in rodents. Studies in progress focus on the peculiar modes of DNA segregation, synthesis and genomic organization so clearly distinct from those in eukaryotic cells. The methods of observation are straightforward and can be readily practiced in any biology laboratory. Interested researchers are welcome in our laboratories to review the thousands of archived images and to prepare and observe samples of their own.
Figures and Tables
Figure 1 Symmetrical forms of amitosis in human fetal tissue. (A–E) “Kissing” bell symmetrical amitoses. Feulgen purple stained nuclei, arranged in order of separation of condensed DNA rings at the mouths of bell shaped nuclei. They are usually observed near midline of syncytia, but also as free single cells in earliest gestational samples, 5–7 wks [(A), brain 13 wks; (B) gut 5–7 wks; (C) brain 9 wks; (D and E) spinal cord, 9 wks]. (F–K) “Stacking cup” symmetrical amitoses. Feulgen purple stained nuclei, arranged in order of separation of condensed DNA rings at bell mouths. Usually observed throughout syncytia of 5–12 wks, but also as free single cells in post-syncytial phase, 12 weeks in fetuses to juvenile period. (L) Quantitative Feulgen estimates of DNA content (picogm DNA) as a function of average separation of condensed DNA rings at bell mouths for “stacking cup” symmetrical amitoses (as arrowed in ‘G’): Green area, separation by ∼1–2 µm; pink area, separation by ∼2–6 µm; blue area, separation ∼6–10 µm and olive area, separation 10 µm. DNA of condensed rings, ∼0.6 picogm, is doubled first in both forms of amitoses followed by ring separation [(F–I), brain 9 wks; (J) gut 5–7 wks; (K) spinal cord 9 wks]. Scale bar, 5 µm.
![Figure 1 Symmetrical forms of amitosis in human fetal tissue. (A–E) “Kissing” bell symmetrical amitoses. Feulgen purple stained nuclei, arranged in order of separation of condensed DNA rings at the mouths of bell shaped nuclei. They are usually observed near midline of syncytia, but also as free single cells in earliest gestational samples, 5–7 wks [(A), brain 13 wks; (B) gut 5–7 wks; (C) brain 9 wks; (D and E) spinal cord, 9 wks]. (F–K) “Stacking cup” symmetrical amitoses. Feulgen purple stained nuclei, arranged in order of separation of condensed DNA rings at bell mouths. Usually observed throughout syncytia of 5–12 wks, but also as free single cells in post-syncytial phase, 12 weeks in fetuses to juvenile period. (L) Quantitative Feulgen estimates of DNA content (picogm DNA) as a function of average separation of condensed DNA rings at bell mouths for “stacking cup” symmetrical amitoses (as arrowed in ‘G’): Green area, separation by ∼1–2 µm; pink area, separation by ∼2–6 µm; blue area, separation ∼6–10 µm and olive area, separation 10 µm. DNA of condensed rings, ∼0.6 picogm, is doubled first in both forms of amitoses followed by ring separation [(F–I), brain 9 wks; (J) gut 5–7 wks; (K) spinal cord 9 wks]. Scale bar, 5 µm.](/cms/asset/1d4849ee-81a7-42f9-ab32-11b0e42fabe4/kogg_a_10909632_f0001.gif)
Figure 2 Bell shaped, metakaryotic nuclei in syncytia. (A) Feulgen purple stained stacking bell-to-bell symmetrical amitotic fission of bell shaped nuclei (upper) imposed over green Feulgen fluorescent image (lower) showing sarcomeric striations (arrowed) of syncytial walls (human fetal spinal cord, 9 wks). (B) Feulgen purple stained bell shaped nuclei in syncytium illustrating variety of nuclear dimensions (human fetal gut, 7 wks). (C) Green fluorescent image of a single syncytium with bell shaped nuclei (left image) and the same image merged with the image of Feulgen purple stained nuclei showing positions of the nuclei in syncytium (human fetal gut, 7 wks). Note, both “kissing cup” and “stacked cup” amitotic figures and bell shaped nucleus apparently emerging from end of syncytium. (D) Section of a Feulgen purple stained syncytium showing a portion of a larger series of eight condensed spherical nuclei (arrowed) between bell shaped nuclei interpreted as the result of synchronous asymmetrical amitoses (human fetal gut, 7 wks). Scale bar, 5 µm.
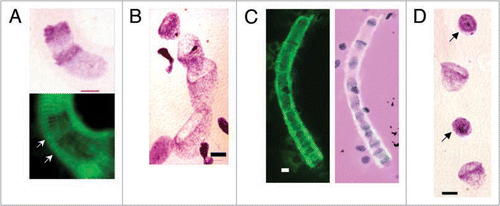
Figure 3 Syncytial clusters in different human fetal meta-organs. (A) Cardiac unstriated muscle at 10 weeks: Feulgen purple stained tubular syncytia (left low corner, arrows) with bell shaped nuclei in central position (zoomed image, left upper corner, 100x). Tubular syncytia are striated with bell shaped nuclei and thus distinct from differentiated cardiac unstriated muscle fibers (arrows) with closed elliptical nuclei aligned with surface of muscle fiber. (B) Skeletal striated muscle of thigh at 10 weeks: Feulgen purple stained tubular syncytia (arrows) lying in parallel with striated muscle fibers (arrowed) with sarcomeric structures with closed elliptical nuclei apparently externally associated to muscle fibers. (C) Same image as (B) merged with green Feulgen fluorescent image. Fluorescence from metakaryotic tubular syncytia is more intense than from differentiated muscle fibers. (D) Spinal cord ganglia at 9 weeks. Multiple clusters of tubular syncytia with 16 bell shaped nuclei each, green Feulgen fluorescence superimposed on purple stained Feulgen image. (E) Brain at 9 weeks. Clusters of syncytia with ∼16–32 bell shaped nuclei each stained as in (C and D). Scale bar, 100 µm.
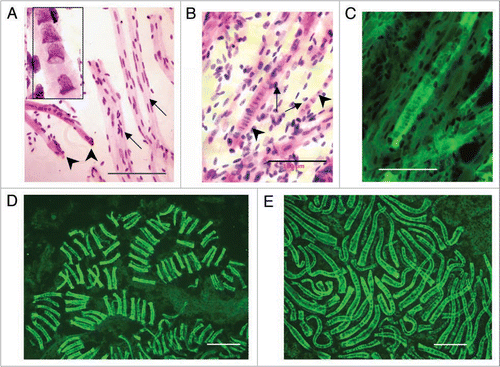
Figure 4 Cells with bell shaped nuclei in post-syncytial stage in human fetal tissues. (A) Syncytial narrowing and fragmentation (arrow), brain, 13 wks. (B) Early stage of amitotic symmetrical stacking bell-to-bell nuclear fission (arrow) in narrowing syncytium (upper) and green Feulgen fluorescence image superimposed on purple stained Feulgen image (lower), brain, 13 wks. (C) Bright green Feulgen fluorescence delineates distribution of cytoplasms attached to bell shaped nuclei in small intestinal villus (11 wks). All cells with “closed” nuclear morphologies observed to date emit relatively little cytoplasmic green Feulgen fluorescence. (D) Feulgen purple stained post-syncytial bell shaped nucleus among closed nuclei (upper) and green Feulgen fluorescence superimposed on purple stained Feulgen image (lower). Note the intense Feulgen fluorescence of the post-syncytial, balloon-like cytoplasm emanating from bell shaped nucleus (human fetal colon, 12 wks). (E) Feulgen purple stained asymmetrical “bell-to-cigar” nuclear fission in post-syncytial single cell with bell shaped nucleus (left) and green Feulgen fluorescence superimposed on purple stained Feulgen image (right), small intestine, 12 wks. (F) Feulgen purple stained asymmetrical ‘bell-to-oval’ nuclear fission in post-syncytial single cell with bell shaped nucleus (left) and green Feulgen fluorescence superimposed on purple stained Feulgen image (right), small intestine, 12 wks. Scale bars, 10 µm except 40 µm in (C).
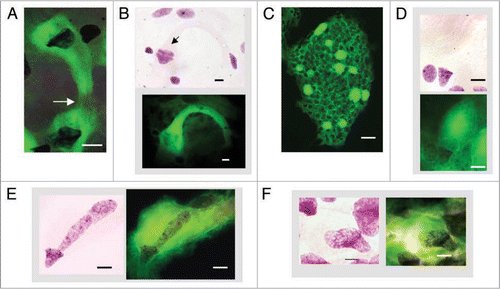
Figure 5 Bell shaped metakaryotic nuclei in development of animals and a plant. (A) Human gut, 7 weeks. (B) Rat, rib cage muscle, 18 days. (C) Mouse spinal cord ganglia, 16.5 days. (D) Plant Arabidopsis, embryonic stem, 1.5 days post germination. (A–D) Feulgen purple stained nuclear DNA. Note condensed, ring-like DNA at bell's mouth in all species. (E) Cluster of tubular syncytia in mouse fetal spinal cord ganglia at 14.5 days, green Feulgen fluorescence. (F) Syncytial bell shaped nuclei of mouse fetal spinal cord ganglia, 16.5 days. Green Feulgen fluorescence (left) superimposed on purple stained Feulgen image (right). (G) Symmetrical, ‘bell-to-bell’ nuclear fission in mouse spinal cord ganglia, 14.5 days. (H) Symmetrical, ‘bell-to-bell’ nuclear fission in rat rib cage muscle, 18 days. (I) Asymmetrical, ‘bell-to-sphere’ nuclear fission delineated in syncytium of a mouse spinal cord ganglia, 14.5 days, phase contrast. (J) Asymmetrical, ‘bell-to-condensed sphere’ nuclear fission in mouse myocyte cell line (Dr. J. Sherley, Boston Biomedical Research Institute, Watertown, MA). Scale bar, 5 µm, save in (D), 2 µm and in (E), 100 µm.
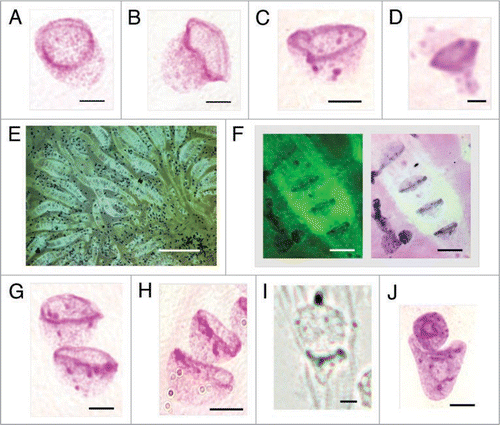
Figure 6 Bell shaped nuclei observed with various techniques in tissues, tumor and cell culture preparations. (A) DAPI stained DNA (blue) syncytial bell shaped nuclei in human fetal spinal cord ganglia, 8–9 wks. (B) DAPI staining (blue) and pan-telomeric FISH labeling (Cy3, red) of bell shaped nuclei in mouse fetal spinal cord ganglia tissue, 16.5 days. (C) DAPI stained syncytia with multiple bell shaped nuclei as seen in 3D imaging [Z-stack, ‘Apotome’], human fetal spinal cord ganglia, 9 wks. (D) High resolution 3D image of DAPI stained bell shaped nucleus and pan-centromeric FISH staining (FITC green), human colon adenocarcinoma, 68 yrs. (E) DAPI stained bell shaped nucleus dividing asymmetrically (bell to cigar shaped nucleus) in human colon adenocarcinoma, 68 yrs. [Arrows indicate the walls of the balloon shaped cytoplasm through which new eukaryotic nuclei migrate]. (F) DAPI stained bell shaped nucleus dividing symmetrically with segregation of pair of chromosomes 18 stained by FISH (FITC green), fetal spinal cord ganglia, 8–9 wks. (G) H&E staining of metakaryotic extra syncytial cell, colon adenocarcinoma, 68 yrs. (H) H&E staining of syncytial bell shaped nuclei, spinal cord, 8–9 wks. (I) Acridine orange stained syncytial bell shaped nuclei, spinal cord, 8–9 wks. (J and L) Feulgen stained DNA (purple) bell shaped nuclei appearing in human adenocarcinoma cell line. (K and M) Feulgen fluorescent (green) images of (J and L), respectively showing unidentified fluorescent material in cytoplasm of all cells of bell shape and with a small fraction of cells with spherical nuclei. (N) Feulgen stained bell shaped nucleus as seen in 30 microns snap frozen tissue section of human colon polyp, 27 yrs. Arrow indicates position of the nucleus relativel to aberrant crypt. Bar scales, 5 µm (A–N) except 50 µm in (H).
![Figure 6 Bell shaped nuclei observed with various techniques in tissues, tumor and cell culture preparations. (A) DAPI stained DNA (blue) syncytial bell shaped nuclei in human fetal spinal cord ganglia, 8–9 wks. (B) DAPI staining (blue) and pan-telomeric FISH labeling (Cy3, red) of bell shaped nuclei in mouse fetal spinal cord ganglia tissue, 16.5 days. (C) DAPI stained syncytia with multiple bell shaped nuclei as seen in 3D imaging [Z-stack, ‘Apotome’], human fetal spinal cord ganglia, 9 wks. (D) High resolution 3D image of DAPI stained bell shaped nucleus and pan-centromeric FISH staining (FITC green), human colon adenocarcinoma, 68 yrs. (E) DAPI stained bell shaped nucleus dividing asymmetrically (bell to cigar shaped nucleus) in human colon adenocarcinoma, 68 yrs. [Arrows indicate the walls of the balloon shaped cytoplasm through which new eukaryotic nuclei migrate]. (F) DAPI stained bell shaped nucleus dividing symmetrically with segregation of pair of chromosomes 18 stained by FISH (FITC green), fetal spinal cord ganglia, 8–9 wks. (G) H&E staining of metakaryotic extra syncytial cell, colon adenocarcinoma, 68 yrs. (H) H&E staining of syncytial bell shaped nuclei, spinal cord, 8–9 wks. (I) Acridine orange stained syncytial bell shaped nuclei, spinal cord, 8–9 wks. (J and L) Feulgen stained DNA (purple) bell shaped nuclei appearing in human adenocarcinoma cell line. (K and M) Feulgen fluorescent (green) images of (J and L), respectively showing unidentified fluorescent material in cytoplasm of all cells of bell shape and with a small fraction of cells with spherical nuclei. (N) Feulgen stained bell shaped nucleus as seen in 30 microns snap frozen tissue section of human colon polyp, 27 yrs. Arrow indicates position of the nucleus relativel to aberrant crypt. Bar scales, 5 µm (A–N) except 50 µm in (H).](/cms/asset/8b2c6c38-329a-44ff-b834-716e1f8320e8/kogg_a_10909632_f0006.gif)
Acknowledgements
This work was supported primarily by private family funds (E.V.G., W.G.T.) and internal funds of the Department of Surgery, Medical College of Wisconsin (A.T.-M., M.M., M.A.G.). J.N.F. and F.D. were supported by internal funds of the Leiden University Medical Centre. We gratefully acknowledge correspondence with Dr. R. Drummond, Heatherton Hospital, Cheltenham, Victoria, Australia of value in recognizing the difficulties in communicating evidence at odds with generally accepted views on organogenesis and stem cells and for providing us with many citations of studies and commentary regarding amitosis in organ development of the past 120 years. We thank Dr. T. Sato, Medical College of Wisconsin for the two pediatric tissue samples in which bell shaped nuclei were detected.
References
- Gostjeva EV, Zukerberg L, Chung D, Thilly WG. Bell shaped nuclei dividing by symmetrical and asymmetrical nuclear fission have qualities of stem cells in human colonic embryogenesis and carcinogenesis. Cancer Genet Cytogenet 2006; 164:16 - 24
- Gostjeva EV, Thilly WG. Stem cell stages and the origins of colon cancer: a multi-disciplinary perspective. Stem Cell Rev 2005; 1:243 - 252
- Wilson EB. The Cell in Development and Inheritance 1896; New York, MacMillan
- Child CM. Amitosis as a factor in normal and regulatory growth. Anat Anz 1907; 30:271 - 297
- Stough HB. Further studies of modified mitosis. J Morphol 1935; 58:221 - 256
- Boveri T, Zellenstudien VI. Jena 1907;
- Poulsen J, Tos M. Goblet cells in the developing human nose. Acta Otolaryngol 1975; 80:434 - 442
- Perreault N, Beaulieu JF. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp Cell Res 1998; 245:34 - 42
- Masuda K, Ikeda H, Kasai K, Fukuzawa Y, Nishimaki H, Takeo T, et al. Diversity of restitution after deoxycholic acid-induced small intestinal mucosal injury in the rat. Dig Dis Sci 2003; 48:2108 - 2115
- Maezato K, Nishimaki T, Oshiro M, Yamashiro T, Sasaki H, Sashida Y. Signet-ring cell carcinoma of the esophagus associated with Barrett's epithelium: report of a case. Surg Today 2007; 37:1096 - 1101
- Cotter DR, Honavar M, Everall I. Focal cortical dysplasia: a neuropathological and developmental perspective. Epilepsy Res 1999; 36:155 - 164
- Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, et al. Normal myoblast fusion requires myoferlin. Development 2005; 132:5565 - 5575
- Mintz B, Baker WW. Normal mammalian muscle differentiation and gene control of isocitrate dehydrogenase synthesis. Proc Natl Acad Sci USA 1997; 58:592 - 598
- Cohnheim J. Congenitales, quergestreiftes Muskelsarkon der ieren. Virchows Arch 1875; 65:64
- Cohnheim J. Vorelesungen uber allgemeine Pathologie. Ein Handbuch fur Artzte und Studierende 1877–80; 691:Berlin Hirchswald 1 - 2
- Sudo H, Li-Sucholeiki XC, Marcelino LA, Gruhl AN, Zarbl H, Willey JC, et al. Distributions of five common point mutants in the human tracheal-bronchial epithelium. Mutat Res 2006; 596:113 - 127
- Sudo H, Li-Sucholeiki XC, Marcelino LA, Gruhl AN, Herrero-Jimenez P, Zarbl H, et al. Fetal-juvenile origins of point mutations in the adult human tracheal-bronchial epithelium: Absence of detectable effects of age, gender or smoking status. Mutat Res 2007; 646:2540
- Coller HA, Khrapko K, Herrero-Jimenez P, Vatland JA, Li-Sucholeiki XC, Thilly WG. Clustering of mutant mitochondrial DNA copies suggests stem cells are common in human bronchial epithelium. Mutat Res 2005; 578:256 - 271
- Brash DE, Ponten J. Skin precancer. Cancer Surv 1998; 32:69 - 113
- Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci USA 1996; 93:14025 - 14029
- Sankaranarayanan K, Chakraborty R. Ionizing radiation and geneticrisks XI. The doubling dose estimates from them id-1950s to the present and the conceptual change to the use of human data on spontaneous mutation rates and mouse data on induced mutation rates for doubling dose calculations. Mut Res 2000; 453:107 - 127
- Okuyama S, Mishina H. Evolution of Cancer 1990; Tokyo, Japan University of Tokyo press
- Okuyama S. Probable binary fission in a Reed-Sternberg cell. Tohoku J Exp Med 1991; 164:247 - 249
- Fomina J, Darroudi F, Boei JJ, Natarajan AT. Discrimination between complete and incomplete chromosome exchanges in X-irradiated human lymphocytes using FISH with pan-centromeric and chromosome specific DNA probes in combination with telomeric PNA probe. Int J Radiat Biol 2000; 76:80713