Abstract
Biodegradable polymers with high mechanical strength, flexibility and optical transparency, optimal degradation properties and biocompatibility are critical to the success of tissue engineered devices and drug delivery systems. Most biodegradable polymers suffer from a short half life due to rapid degradation upon implantation, exceedingly high stiffness, and limited ability to functionalize the surface with chemical moieties. This work describes the fabrication of microfluidic networks from poly(ester amide), poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) (APS), a recently developed biodegradable elastomeric poly(ester amide). Microfluidic scaffolds constructed from APS exhibit a much lower Young’s Modulus and a significantly longer degradation half-life than those of previously reported systems. The device is fabricated using a modified replica-molding technique, which is rapid, inexpensive, reproducible, and scalable, making the approach ideal for both rapid prototyping and manufacturing of tissue engineering scaffolds.
Polymeric Scaffolds for Tissue Engineering
One of the principal challenges in the field of tissue engineering has been the inability to produce tissue constructs with an intrinsic vasculature, a requirement for most complex vascularized tissues and organs.Citation1,Citation2 Approaches to address this challenge include the use of angiogenic growth factors in tissue engineering scaffolds,Citation3 seeding scaffolds with stem cells to generate micro-vasculatureCitation4,Citation5 as well as the formation of structures comprising three-dimensional microfluidic networks pre-seeded with endothelial cells.Citation6–Citation8 For this latter approach, one of the most important considerations is the selection of an appropriate polymeric scaffolding material, possessing suitable mechanical, chemical and biodegradation characteristics for the specific application. Initial development of microfluidic constructs for vascularized tissues utilized Poly(Di-Methyl-Siloxane) (PDMS),Citation6 an elastomer commonly employed in lab-on-a-chip applications. While PDMS is optically transparent, flexible and biocompatible,Citation9 it is not biodegradable and the surface chemistry can be unstable,Citation10 rendering it unsuitable for implantable applications. A series of biodegradable polymers have been explored as scaffolding materials for vascularized tissue engineering, including PLGA,Citation11 PGSCitation12,Citation13 and silk fibroin.Citation14,Citation15 However, for applications requiring flexible yet strong scaffolds with long half-lives, these materials exhibit either non-ideal mechanical properties (PLGA, silk) or excessively rapid degradation (PGS), highlighting the need for degradable polymer substrates with tunable properties for engineered tissues and implants.
To develop a longer-lasting scaffold substrate than PGS while preserving its excellent chemical and mechanical properties, a new class of biodegradable elastomers, poly(ester amide), poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) (APS), has been developed.Citation16 This class of APS materials is tunable across a wide range of chemical compositions, resulting in degradation times between 6 weeks and one year, which are governed by a range of different degradation mechanisms. The material has been shown to be highly biocompatible, with primary hepatocyte culture showing extended cell functionality without requiring deposition of protein coatings on the surface, and amenable to nanostructuring to provide surface topographic features representative of the cell microenvironment.Citation17 In this work, we present new data on the APS polymer system relevant for tissue engineering applications, including microfabrication results for microfluidic channel networks for comparison with existing degradable polymer systems. These data demonstrate that the APS system represents a novel class of polymers for the development of microfluidic scaffolds for tissue engineering applications.
Background
Biodegradable polymers.
Among the commonly used biodegradable polymers, polyesters and polyamides have shown great potential in part because of their controlled degradation properties. Both typically degrade via hydrolysis, and polyester generally degrades comparatively faster than polyamides.Citation18 Based on these observations, a rationale for the development of APS was generated. For many tissue engineering and implantable device applications, a long half-life and durable properties are desired. The class of APS polymers was developed as a mixture of polyester and polyamide, providing a tunable and extended half-life. It was reported to have mass loss of around 13% after 20 weeks,Citation16 a much slower degradation rate than PGS, which had a mass loss of around 17% after 60 days (roughly 9 weeks).Citation19
As previously mentioned, existing biodegradable polymers have properties optimized for specific clinical applications, but many of the commonly used materials degrade fairly rapidly.Citation20 Another issue seen especially in polyamides is exceedingly high stiffness (e.g., nylons), which is a challenge for applications requiring flexibility and malleability based on surgical considerations. Finally, many biodegradable polymers have a limited ability to attach chemical moieties to the surface, a serious problem because of the need for chemical functionalization in order to facilitate cell attachment. Compared with existing biodegradable polymers, the strengths of APS include a slower, tunable degradation rate, the production of bioabsorbable monomers, excellent mechanical properties and compatibility with cell culture, all of which suggest that APS is an excellent polymer substrate for tissue engineering use.
Specific properties of APS.
The polymer investigated here is from a class of new biodegradable elastomeric poly(ester amide)s, poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate)s (APS),Citation16 which have been developed as an alternative to the traditional crosslinked aliphatic polyesters to address the above-mentioned drawbacks. It is composed of nontoxic, inexpensive monomers commonly used in biomedical systems. The relatively low Young's modulus and tunable biodegradation half-life enable the material to attain biomimetic mechanical properties similar to those of extra-cellular matrix (ECM), and to have compatibility with dynamic mechanical environments. The participation of the polymer in hydrogen bonding also allows for subsequent conjugation reactions and cell seeding and adhesion. The hydrolysis rate and elastic modulus of the polymer can both be tuned using different amide percentage. It is also susceptible to enzymatic degradation, where the degradation rate of both the ester groups and amide groups can be individually and precisely triggered using different enzymes.
Biodegradable microfluidics.
One of the principal challenges in tissue engineering is the formation of a vasculature to support oxygen and nutrient transport within the growing tissue. One avenue for achieving this goal is the formation of a microfluidic network within the tissue engineering scaffold; an initial proof of principle for this concept was demonstrated using nondegradable PDMS as the substrate for endothelialized microfluidic networks.Citation6 Early demonstrations of biodegradable microfluidic devices were reported by Armani and Liu,Citation21 King et al.Citation11 and Liu and Bhatia.Citation22 The former report required the insertion of a nondegradable metallic layer for bonding the degradable PLGA films; the latter two were constructed solely from biodegradable PLGA. These structures suffered from excessive mechanical stiffness, spurring the development of biodegradable elastomers such as PGS. The relatively short half-life of PGS has led to further development of APS as a tunable biodegradable elastomer for tissue engineering applications; here we present the first report of a microfluidic network formed from the APS polymer.
The technical approach here is similar to that reported for PLGA,Citation11 PGSCitation12,Citation13 and silk fibroin.Citation14,Citation15 Briefly, a simple microchannel network design was generated based on principles of microvascular flow, pressure and wall shear stress.Citation23 This microchannel network is based upon design principles including uniform flow and distribution of oxygen and nutrients throughout a scaffold layer, as well as physiologic levels of pressure drop and wall shear stress within the various vessel sizes in the network. The network was translated into a photolithographic layout for fabrication, with the intent of producing a single-layer microvascular network.
The process begins with silicon master mold fabrication using SU-8 photolithography, with minimum channel dimensions of 35 microns in depth and 30 microns in width, on the order of the size scale of capillaries. The channel depth is set by the thickness of the SU-8 photolithographic layer, and is constant throughout the entire 2D network. The channel width is varied across the network, to reflect the size scales of the various vessels in the arterial and venous circulation. Once the lithographic pattern is formed on the silicon master surface, the wafer must be prepared for polymer molding by depositing a surface coating that can be removed sacrificially to release the molded polymer layer. For PGS, the optimal surface coating was found to be sucrose dissolved in water, but process development with APS determined that the temperatures involved in curing resulted in unacceptably high levels of sucrose caramelization during the process. Therefore, we investigated alternative sacrificial sugar coatings and determined that maltose has a caramelization temperature about 20°C higher than sucrose. Therefore, maltose coatings were deposited onto the silicon masters to assist in delamination of APS films, and APS was cast onto the silicon master molds and then lifted off as a free-standing film. A channel layer was bonded to a flat APS layer and the layers joined at elevated temperature and pressure. The challenge for layer bonding is to form a strong, irreversible and leakproof bond between films, without raising the temperature or pressure so high as to deform or collapse the microchannels or other high resolution structures. In order to prepare the bonded devices for future flow testing and cell seeding, silicone tubing was attached to the APS microfluidic scaffold to demonstrate flow through the network at physiological pressures (0–180 mmHg).
Results
Mechanical testing.
Mechanical properties of APS and PGS have been measured and are summarized in . Along with the Young's modulus and elasticity, the synthesis properties and degradation rate are characterized for both materials. The table indicates that APS is slightly stronger and less elastic than PGS, but that the synthesis process is more controlled and the degradation rate much slower.
As shown in , it was found that the curing time, the curing temperature and the thickness of scaffold are all significant factors in the formation of APS scaffolds. It was shown that the longer APS curing time and higher curing temperature makes scaffolds with higher tensile strength. It was also found that there is an exponential relationship between elastic modulus and scaffold thickness across a range of curing times. Regardless of the curing temperature, the elasticity decreased exponentially as thickness was increased for fixed curing times. There was also a linear relationship between tensile strength and the UTS of APS.
Microfluidic scaffolds.
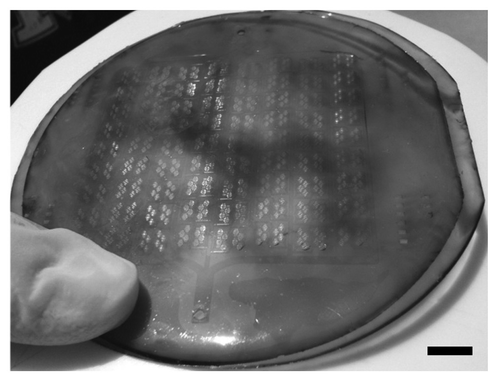
As shown in , a simple construct comprising an APS microfluidic channel was utilized for this first demonstration. The APS microchannel network layer was placed against a flat APS sheet, with a PDMS structural support placed underneath the flat sheet. The PDMS structural support was there simply for tubing stability but is not required, since the future embodiment of the device will incorporate tubing directly connected to the APS layers. The microfabricated channels in APS are shown in , where the film exhibits high fidelity reproduction of the silicon master channel features down to the 30 micron feature size. There is no evidence of channel distortion due to the de-molding process, although SEM analysis is now underway to determine the precise feature dimensions relative to those on the master wafer. Finally, shows the bonded APS construct with the flat film and the microchannel film, each at the full 100 mm diameter size, laminated together in preparation for flow testing and eventual cell seeding. The quality of the layer bonding across the full wafer size appears to be excellent.
Discussion and Conclusion
The concept of biodegradable microfluidic devices was first introduced using PLGA in by Armani and LiuCitation21 and King et al.Citation11 but these device constructs exhibited a high degradation rate, low elasticity and concerns regarding immune and inflammatory response in bulk format. More recently, Fidkowski et al.Citation12 and Bettinger et al.Citation13 constructed PGS-based devices that exhibit much more desirable mechanical properties for implantation, but the relatively high degradation rate of the polymer limits its applicability for certain long-lasting applications as an implant. In the present study, we report on the development of a specific process for biodegradable microfluidic devices constructed from APS, an alcohol-based poly(ester amide) elastomer, which possesses a much lower degradation rate but retains elastomeric properties for tissue scaffold applications. The process reported here is capable of forming microfluidic channels on a wafer-scale with strong layer-to-layer bonds and well-preserved microscale architecture. As the biocompatibility of APS in vivo was also shown in the study done by Bettinger et al.,Citation24 it was demonstrated that the material could be used for resorbable tissue engineering devices for the purpose of drug delivery and regenerative medicine. The next steps for this investigation will focus on the establishment of robust flow conditions within the devices, seeding of endothelial cells to form a functional microvascular network and implantation studies to explore in vivo biocompatibility, host integration and long-term degradation properties for this scaffolding material.
Materials and Methods
APS synthesis.
Synthesis of poly(1,3-diamino-2-hydroxy-propaneco-glycerol sebacate) elastomers. All materials were purchased from Sigma Aldrich (St. Louis, MO) and used as received unless otherwise specified. A round bottom flask was charged with 0.06 mol of 1,3-diamino-2-hydroxy-propane (DAHP), 0.03 mol glycerol (G) and 0.09 mol of sebacic acid (SA) to produce a molar ratio of 2:1:3 of DAHP:G:SA, respectively. The reactants were heated under an argon blanket at 130°C for 3 h. The pressure was then dropped to approximately 50 mTorr and the contents were allowed to react for 10 h at 130°C. The product was then stored under a desiccant environment until further use. The product was spread onto silicon wafer molds and glass slides and cured at 170°C at approximately 50 mTorr for either 24 h or 48 h. Film thicknesses of either 0.5 mm or 1 mm were achieved by applying the reaction product at surface densities of 100 mg/cm2 and 200 mg/cm2, respectively.
PGS synthesis.
PGS pre-polymer was synthesized by step growth polymerization of 0.1 mole each of glycerol (Aldrich, Milwaukee, WI) and sebacic acid (Aldrich) as previously described.Citation19 The resulting product was stored under a desiccant environment as is for later use. The product was spread onto silicon wafer molds and glass slides and cured at 160°C at approximately 50 mTorr for times ranging from 8 to 24 h.
Both APS and PGS films were delaminated in ddH2O at 70°C for 18 h. Sol was removed by incubating polymer films in 100% ethanol for 24 h followed by washing and incubation with ddH2O. Subsequent characterization techniques were performed on sol-free samples only.
Mechanical testing.
The thicknesses of APS films were determined by measuring at the five separate points across the sample with a dial gauge (L.S. Starrett, Athol, MA). The samples were cut to size (approximately 2.0 × 20.0) mm2 and mounted on an Electroforce ELF 3200 mechanical tester (Bose-Enduratec, Framingham, MA) with a 250 g load cell (model 31-1435-03; Sensotech, Columbus, OH). The samples were elongated to failure.
Microfabrication of microfluidic scaffolds.
The silicon mold was designed and created as previously described by Bettinger et al.Citation13 Prior to replica molding of APS, a sacrificial maltose release layer was spin-coated on the silicon master. Photolithographically patterned silicon masters were cleaned using piranha solution (Mallinckrodt, St. Louis, MO) and oxygen plasma-cleaned (March, St. Petersburg, FL) at 250 mTorr and 200 W for 45 seconds. A 90% (w/w) solution of maltose (Sigma, St. Louis, MO) in water was spin-coated at 2,500 revolutions per minute for 30 s. The maltose layer was pre-baked on a hot plate at 95°C for 120 seconds. 7.00 ± 0.05 g of APS prepolymer was melted at 170°C and applied to the wafers for replica molding and smooth sheet formation. The thickness of the final APS film could be adjusted simply by varying the mass of prepolymer used. The prepolymer was cured at 170°C for 48 h under 50 mTorr of vacuum, which produced a crosslinked sheet in which a portion of the hydroxyl and carboxylic acid functional groups remained. The APS sheets were delaminated by statically incubating the polymer-master system in doubly distilled water (ddH2O) at 60°C for 24 h beginning immediately after polymer curing. Diffusion of water between the polymer/silicon interfaces led to maltose dissolution and eventual delamination. Sheets were trimmed and punched to achieve appropriate fluidic connections between layers. Microfluidic layers were stacked, aligned and bonded together simultaneously by oxygen plasma treatment for surface activation, followed by curing the polymer at 170°C for 24 h under 50 mTorr of vacuum. An additional layer of PDMS was laminated onto the device for tubing stabilization. Once the final curing step was completed, silicone tubing (1/16 in. inner diameter, 1/8 in. outer diameter, Cole-Parmer) was inserted into the devices in a sterile environment. Luer-Lok connections were inserted into the tubing, and the base of the connections was sealed with epoxy (McMaster-Carr). In some cases, additional PDMS structures were added to the inlet and outlet to prevent dissociation of the tubing from the device.
Abbreviations
| APS | = | poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) |
| DAHP | = | 1,3-diamino-2-hydroxy-propane |
| ddH2O | = | double deionized water |
| ECM | = | extra-cellular matrix |
| G | = | glycerol |
| PDMS | = | poly(di-methyl-siloxane) |
| PGS | = | poly(glycerol sebacate) |
| PLGA | = | poly(lactic-co-glycolic acid) |
| SA | = | sebacic acid |
| UTS | = | ultimate tensile strength |
Figures and Tables
Figure 1 The variation between thicknesses of scaffolds and mechanical strength (regardless of the curing temperature) exhibited exponential correlation. With the same amount of curing time, the elasticity decreases exponentially as thicknesses increases.
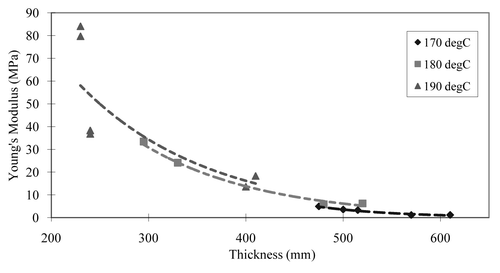
Figure 2 A schematic diagram for the design of the microfluidic device built in APS. Top material is the flat PDMS scaffold for tubing stabilization. Middle scaffold is the flat APS scaffold. Bottom scaffold is the patterned APS scaffold.
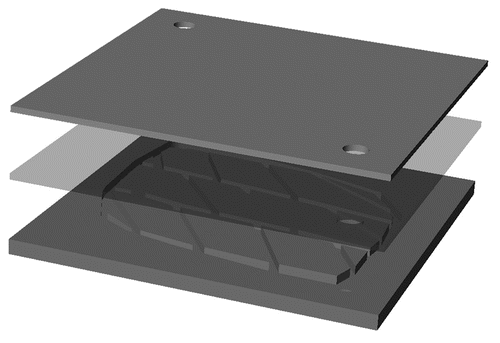
Figure 3 Microfluidic channels mimicking blood vessel bifurcated networks built in APS. Scale bar in the image is 100 µm.
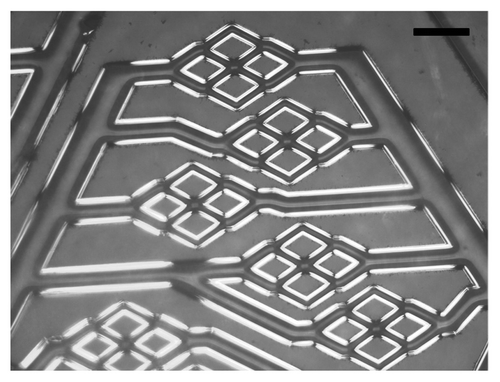
Table 1 Comparison between PGS and APS
Acknowledgements
Funding for this work was provided by C.S. Draper's laboratory and is gratefully acknowledged. We are indebted to G.C. Engelmayr, L.E. Freed, J. Hsiao and T. Kniazeva for many useful discussions and assistance with the instrumentation and polymer synthesis.
References
- Langer R, Vacanti JP. Tissue engineering. Science 1993; 260:920 - 926
- Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA 2006; 103:2480 - 2487
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol 2001; 19:1029 - 1034
- Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 2002; 99:4391 - 4396
- Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol 2007; 25:317 - 318
- Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication technology for vascularized tissue engineering. Biomed Microdevices 2002; 4:167 - 175
- Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvascular Res 2006; 71:185 - 196
- McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci USA 2006; 103:11461 - 11466
- Whitesides GM, Stroock AD. Flexible methods for microfluidics. Physics Today 2001; 54:42 - 48
- Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006; 6:1484 - 1486
- King KR, Wang CCJ, Kaazempur-Mofrad MR, Vacanti JP, Borenstein JT. Biodegradable microfluidics. Adv Mater 2004; 16:2007
- Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang YD. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng 2005; 11:302 - 309
- Bettinger CJ, Weinberg EJ, Kulig KM, Vacanti JP, Wang YD, Borenstein JT, et al. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv Mater 2006; 18:165
- Bettinger CJ, Cyr KM, Matsumoto A, Langer R, Borenstein JT, Kaplan DL. Silk fibroin microfluidic devices. Adv Mater 2007; 19:2847 - 2850
- Borenstein JT, Tupper MM, Mack PJ, Weinberg EJ, Khalil AS, Hsiao J, et al. Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomed Microdevices 2010; 12:71 - 79
- Bettinger CJ, Bruggeman JP, Borenstein JT, Langer RS. Amino alcohol-based degradable poly(ester amide) elastomers. Biomaterials 2008; 29:2315 - 2325
- Bettinger CJ. Synthesis and microfabrication of biomaterials for soft-tissue engineering. Pure Appl Chem 2009; 81:2183 - 2201
- Wachsmuth ED, Fritze I, Pfleiderer G. An aminopeptidase occurring in pig kidney. II. A study on the mechanism of the hydrolysis. Biochemistry 1966; 5:175 - 182
- Wang YD, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol 2002; 20:602 - 606
- Bettinger CJ, Bruggeman JP, Borenstein JT, Langer R. In vitro and in vivo degradation of poly (1,3-diamino-2-hydroxypropane-co-polyol sebacate) elastomers. J Biomed Mater Res A 2009; 91:1077 - 1088
- Armani DK, Liu C. Mircofabrication technology for polycaprolactone, a biodegradable polymer. J Micromechanics Microeng 2000; 10:80 - 84
- Liu Tsang V, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J 2007; 21:790 - 801
- Borenstein JT, Weinberg EJ, Vacanti JP, Kaazempur-Mofrad MR. Khademhosseini A, Borenstein JT, Toner M, Takayama S. Microvascular Engineering: Design, Modeling and Microfabrication. Micro and Nanoengineering of the Cell Microenvironment 2008; Boston Artech House
- Bettinger CJ, Kulig KM, Vacanti JP, Langer R, Borenstein JT. Nanofabricated collagen-inspired synthetic elastomers for primary rat hepatocyte culture. Tissue Eng Part A 2009; 15:1321 - 1329