Abstract
Transplantation therapy for humans is limited by insufficient availability of donor organs and outcomes are complicated by the toxicity of immunosuppressive drugs. Xenotransplantation is a strategy to overcome supply problems. Implantation of tissue obtained early during embryogenesis is a way to reduce immunogenicity of transplants. Insulin-producing cells originating from embryonic pig pancreas obtained very early following initiation of organogenesis [embryonic day 28 (E28)] engraft long-term in non-immune suppressed diabetic rats or rhesus macaques. Recently, we demonstrated engraftment of morphologically similar cells originating from adult porcine islets of Langerhans (islets) in rats previously transplanted with E28 pig pancreatic primordia. Our findings are consistent with induction of tolerance to a cell component of porcine islets induced by previous transplantation of embryonic pig pancreas, a phenomenon we designate organogenetic tolerance. Induction of organogenetic tolerance to porcine islets in humans with diabetes mellitus would enable the use of pigs as islet donors with no host immune suppression requirement. Adaptation of methodology for transplanting embryonic organs other than pancreas so as to induce organogenetic tolerance would revolutionize transplantation therapy.
As reviewed previously in Organogenesis,Citation1,Citation2 transplantation of embryonic organ primordia to replace the function of diseased organs offers theoretical advantages relative to transplantation of either pluripotent ES cells, or of fully differentiated (adult) organs. (1) Unlike ES cells, organ primordia differentiate along defined organ-committed lines. There is no requirement to steer differentiation and no risk of teratoma formation. In the case of embryonic pancreas, the glucose sensing and insulin releasing functions of β cells that differentiate from primordia are functionally linked. (2) The growth potential of cells within embryonic organs is enhanced relative to those in terminally-differentiated organs. (3) The cellular immune response to transplanted primordia obtained early during embryogenesis is attenuated relative to that directed against adult organs. (4) Early organ primordia are avascular. The ability of cellular primordia to attract a host vasculature renders them less susceptible to humoral rejection than are adult organs with donor blood vessels transplanted across a discordant xenogeneic barrier. And (5) organ primordia differentiate selectively. In the case of embryonic pancreas, exocrine pancreatic tissue does not differentiate following transplantation, obviating complications that can result from exocrine components such as the enzymatic autodigestion of host tissues. The finding that it is possible to transplant pig pancreatic primordia to non-human primates with no immunosuppression requirementsCitation3 is very important because it establishes the potential for transplantation in humans with no complications of immunosuppressive therapy.
The physiologies of several porcine organ systems are sufficiently similar to those in humans such that the pig has been suggested to be an organ donor for clinical transplantation.Citation4 Pigs are plentiful, and can be bred in captivity under pathogen-free conditions.Citation5 Porcine insulin works well in humans. Therefore transplanted porcine pancreatic endocrine tissue could be used to treat diabetes in patients. The severity of humoral rejection due to preexisting natural antibodies effectively precludes the use of non-transgenic swine as whole organ (pancreas) donors in primate hosts.Citation1–Citation9 However, isolated cells such as islets of Langerhans (islets) can be transplanted into humansCitation6 or non-human primatesCitation7,Citation8 without initiating humoral rejection. Recent experience with pig to primate islet,Citation7 neonatal islet,Citation8 or hCD46 transgenic pig isletCitation9 transplantation shows that sustained insulin independence can be achieved, but unfortunately only through the use of immune suppressive agents that are not approved for humans or would result in an unacceptable level of morbidity.
We have shown that glucose tolerance can be normalized in streptozotocin (STZ)—diabetic (type 1) LEWCitation10–Citation12 rats or ZDFCitation13 (type 2) diabetic rats within 4 weeks following transplantation in mesentery of pig pancreatic primordia obtained very early during embryogenesis [on embryonic day 28 (E28)—just after the organ differentiates and prior to the time dorsal and ventral anlagen fuse] without host immune-suppression. Rats are rendered permanently independent of a requirement for exogenous insulin to maintain normoglycemia. No circulating rat insulin can be detected in STZ-treated rats. Porcine insulin circulates post-transplantation of E28 pig pancreatic primordia (embryonic pancreas) and levels increase after a glucose load. Cells expressing insulin and porcine proinsulin mRNA with β-cell morphology engraft in host mesentery, mesenteric lymph nodes, liver and pancreas post-transplantation.Citation10–Citation13 Cells originating from E28 pig pancreatic primordia engraft similarly in non immune-suppressed STZ-diabetic rhesus macaques.Citation3
Glucose tolerance can be nearly normalized in non-immune-suppressed diabetic rhesus macaques following transplantation of E28 pig pancreatic primordia (). Exogenous insulin requirements are reduced in transplanted macaques and animals have been weaned off insulin for short periods of time, but not permanently. The most likely explanation for the difference between rats and macaques is that macaques weigh 20 times as much as rats. A STZ-diabetic rat can be rendered normoglycemic lifelong with no exogenous insulin requirement by transplantation of 5–8 pig pancreatic primordia. Extrapolating, it would take 100–160 primordia to render a diabetic rhesus macaque independent of exogenous insulin. This would require the sacrifice of about 7–12 pregnant sows and multiple surgeriesCitation3 with the attendant complications.
In lieu of increasing the number of transplanted primordia in diabetic rhesus macaques, we embarked on a series of experiments to determine whether porcine islets, a more easily obtainable and possibly more robust source of insulin-producing cells, could be substituted in animals rendered tolerant to embryonic pig pancreas. Our first step was to determine using rats, whether engraftment of cells originating from E28 pig pancreatic primordia renders hosts tolerant to the same or similar cell component present in porcine islets from adult swine (adult islets). To this end, we implanted adult porcine islets beneath the renal capsule of rats that previously had been transplanted with E28 pig pancreatic primordia in mesentery.Citation12
Intact porcine islets do not engraft following renal subcapsular implantation. However, a population of cells originating from donor islets with β-cell morphology that express insulin and porcine proinsulin mRNA engraft in kidneys of rats transplanted previously with E28 pig embryonic pancreas. Our observations are consistent with induction of tolerance to a cell component of adult porcine islets by previous transplantation of E28 pig pancreatic primordia in rats.Citation12
is a photograph of a kidney from a STZ-treated rat that had been transplanted with embryonic pig pancreas in mesentery, taken 4 weeks after implantation of islets in kidney. A distinct, whitish well-demarcated graft, which is easily distinguished from the surrounding renal parenchyma is observed (white arrow) along with large intra-capsular venous blood vessels that radiate from the graft out into the renal parenchyma (black arrow).
shows sections from a kidney from a STZ-diabetic rat transplanted previously with embryonic pig pancreas in mesentery and subsequently with pig islets in kidney. Sections are stained using anti-insulin antibodies ( and C) or control serum ( and D). As would be expected for kidney that filters, reabsorbs and secretes insulin, proximal tubules (PT) in are positive (red brown) relative to comparable structures in . Cells that stain for insulin (), but not with control serum () are present in an expanded subcapsular space ( and B; arrowheads). shows a higher magnification of the subcapsular space. The cells that stain positive for insulin (red-brown stain) are polygonal with round nuclei and abundant cytoplasm (arrow), a β-cell morphology.Citation12 Also shown in are sections incubated with antisense () or sense () porcine proinsulin mRNA probes. Hybridization occurs with cells hybridized to the former (arrows), but not the latter probe.
The contralateral (nontransplanted) kidney from a STZ-diabetic rat transplanted previously with embryonic pig pancreas in mesentery and subsequently with pig islets in the ipsilateral kidney is shown in . Sections are stained using anti-insulin antibodies ( and C) or control serum ( and D). A low magnification view of the contralateral kidney shows normal renal morphology with no evidence of engrafted tissue (). A higher power view confirms that, in contrast to what is observed in the transplanted kidney () there is no expansion of the subcapsular space in the contralateral kidney (, arrowheads) and no cells are present with β-cell morphology as shown in . and F shows the subcapsular space of a kidney from a rat implanted with porcine islets four weeks previously with no prior transplantation of E28 pig pancreatic primordia. Sections are stained using anti-insulin antibodies () or control serum (). Insulin staining of proximal tubules (PT) is evident in . The subcapsular space ( arrowheads) is expanded relative to that depicted in and C. However there are no cells with β cell morphology that stain for insulin ().
To provide additional evidence that pig cells are present in kidneys and mesenteric lymph nodes of rats transplanted with pig pancreatic primordia and subsequently with porcine islets, we performed fluorescent in-situ hybridization using a probe specific for the pig X chromosome. Shown in (arrows) are pig X chromosomes in nuclei of cells from a normal porcine pancreas (positive control). (arrows) shows pig X chromosomes in the nuclei of cells in the renal subcapsular space [between a tubule (T) and the renal capsule membrane (RC) in ]; and outside of a germinal center (GC) of a mesenteric lymph node (). There are no cells containing pig X chromosomes in renal cortex from the transplanted kidney (), consistent with the subcapsular localization of the insulin positive and porcine proinsulin mRNA containing cells (), and with species specificity of the pig X chromosome probe (host rats are females).
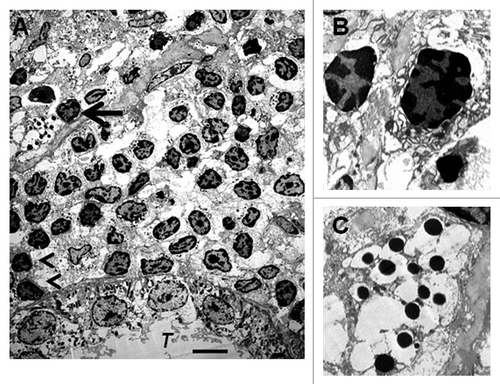
We have shown previously using electron microscopy that cells with β-cell morphology containing granules, some of which have a crystalline core surrounded by a clear space, are present in mesentery of rats following transplantation of E28 pig pancreatic primordia. To ascertain whether similar cells are present in kidneys of rats transplanted with E28 pig pancreatic primordia and subsequently with porcine islets, we performed electron microscopy. Shown in (arrow) is a cell in the renal subcapsular space with β-cell morphology [containing granules (250–400 nm) which have a crystalline core surrounded by a clear space]. A renal tubule (T) is labeled. A mononuclear cell infiltrate in the renal subcapsular space consists predominantly of macrophages. Two are labeled (arrowheads). is a high-power view of two others. shows a high power view of the granules with a crystalline core surrounded by a clear space.
To our knowledge, oursCitation12 is the first report describing sustained survival of β cells following transplantation of porcine islets in non-immune suppressed immune sufficient rodents. In previous studies that did not employ transplantation of E28 pig pancreatic primordia prior to implantation of islets or islet cell clustersCitation14–Citation16 complete destruction of grafts was evident within two weeks. Pre-immunization of rats by subcutaneous injection of porcine islet cell clusters accelerates the rejection of islet clusters transplanted subsequently beneath the renal capsule.Citation16 In contrast, prior transplantation of E28 pig pancreatic primordia in mesentery enables survival of an insulin-expressing cell component of porcine islets implanted subsequently in rat kidneys ().
Schroeder et al.Citation17 define transplantation tolerance as immune unresponsiveness to the transplanted organ, but not to other antigens, in the absence of ongoing immunosuppression. LEW rats transplanted with E28 pig pancreatic primordia retain reactivity to other porcine xenoantigens (E28 pig renal primordia are rejected)Citation11. Thus, our findings are consistent with induction of specific toleranceCitation17 to a cell component (either β cells or a stem cell component that differentiates into insulin-producing cells) of adult porcine islets implanted in LEW rats by previous transplantation of E28 pig pancreatic primordia.
Though not observed following xenotransplantation under all conditions,Citation18,Citation19 host tolerance to early stage pancreatic progenitors has been reported twice previously. Eloy et al. described normalization of glucose post-transplantation of E15, but not E18 embryonic chick pancreas into non-immmune suppressed STZ-diabetic immune competent rats.Citation20 Abraham et al.Citation21 described successful xenoengraftment in multiple organs of human pancreatic islet-derived progenitor cells infused in non-immunosuppressed immune competent mice. It is possible that xenotransplantation of fetal pancreas is particularly suited to induction of tolerance. However, neither Eloy et al.Citation20 nor Abraham et al.Citation21 nor weCitation3,Citation10–Citation13 define an immunological mechanism.
Although the antigenicity of fetal tissues may be less than that of corresponding adult tissues, animal data suggest the reduction is not enough by itself to ensure permanent graft survival.Citation22 Thus, the use of embryonic tissue (pancreas) per se cannot explain our findings. Host immune suppression is required for successful engraftment of embryonic pig pancreas in rodentsCitation18 or non-human primatesCitation19 carried out using methodology different from ours. Therefore, it is likely that one or more differential factors in the methodology we employ is critical for engraftment of embryonic pig pancreas without an immune suppression requirement. Such factors could include the developmental stage of embryos from which primordia are obtained, the number of pancreatic primordia transplanted, the manner in which embryonic pancreas organs are incubated in vitro prior to implantation, the diabetic status of the host, the transplantation site and methodology for securing the implants in place and the stringency by which glucose levels in diabetic hosts are controlled post-transplantation.Citation3,Citation10–Citation13
KorsgrenCitation23 has proposed that rejection of an islet cell xenograft is dependent on two different cellular mechanisms. The first is recognition of pig MHC molecules by cytotoxic lymphocytes via both direct and indirect pathways of antigen recognition, as occurs following rejection of an allograft. The second is an immune response characterized by T cell dependent infiltration of macrophages with histopathological characteristics of delayed-type hypersensitivity. In non-immune suppressed rodents transplanted with porcine islets, the second mechanism predominates. In non-immune suppressed non-human primates, rejection is dominated by the first. However the first process is inhibited in immune suppressed non-human primates and the second, less sensitive to immune suppression, is revealed.
The presence of macrophages beneath the renal capsule of rats transplanted with porcine islets subsequent to E28 pig pancreatic primordia () may reflect the second cellular mechanism described by KorsgrenCitation23 in the context of engraftment of a cell component of porcine islets to which rats have been rendered tolerant by prior transplantation of E28 pig pancreatic primordia. Alternatively, since porcine islets are rejected by immune competent rats without prior transplantation of E28 pig pancreatic primordiaCitation12 the macrophages may represent a population of M2 phenotypeCitation24 partially responsible for the tolerance we observe.
We have proposedCitation25 that transplantation of E28 pig pancreatic primordia in the mesentery and migration of cells to mesenteric lymph nodes and liver recapitulates events that occur during induction of oral tolerance,Citation26–Citation28 the induction of which is dependent on antigen transport via afferent lymphatics into the draining mesenteric lymph nodes.Citation28 In effect, we suggest that heterotopic introduction of embryonic pig pancreas in rat or primate mesentery co-opts the function of the gut associated lymphoid tissues (GALT) a complex, redundantCitation26–Citation28 and phylogenetically ancient systemCitation29,Citation30 of which embryonic pancreas is a part,Citation31 that under normal conditions induces peripheral tolerance to ingested antigens in jawed vertebrates and their descendants.
Interestingly, GALT may have served similarly to prevent an immune response to insulin-producing cells scattered originally in the gut epithelium of primitive vertebratesCitation29,Citation30 and has been proposed to induce tolerance or immune suppression towards islet cell antigens during normal embryonic development.Citation31 Developmentally controlled lymphogenesis establishes a preferential trafficking route from the gut to pancreatic lymph nodes, a GALT component, in which T cells can be activated by antigens drained from the peritoneum and the gastrointestinal tract. Intestinal stress modifies the presentation of pancreatic self-antigens in pancreatic lymph nodes. The convergence of endocrine and intestinal contents at this site may explain the link between an autoimmune pathogenesis for type 1 diabetes and environmental provocation.Citation31,Citation32
Low doses of orally administered antigen induce antigen-specific peripheral tolerance through active suppression of T cells and induction of clonal anergy. High doses induce tolerance by extrathymic deletion of antigen-reactive T cells.Citation33 It was proposed originally, that oral tolerance depends exclusively on antigen uptake by cells within intestinal Peyer's Patches.Citation28 However, recently it has been shown that high dose oral tolerance can be induced in the absence of Peyer's Patches, so long as mesenteric lymph nodes are present.Citation28,Citation34
Given similarities between the host immune responses in rodents and non-human primates to porcine xenograftsCitation23,Citation35 studies are warranted employing non-immune suppressed rhesus macaques transplanted with E28 pig pancreatic primordiaCitation3 as hosts for porcine islets. Such experiments are currently underway in our laboratory. If engraftment of porcine islets takes place in non immune suppressed macaques rendered tolerant by prior transplantation of embryonic pig pancreas, it is likely that comparable engraftment will occur in humans with diabetes mellitus. The ability to employ porcine islet transplants to normalize glucose tolerance in non-immune suppressed patients would widen the applicability for and reduce the toxicity of transplantation therapy for diabetes mellitus.
We have employed embryonic and adult endocrine pancreas to demonstrate a phenomenon, the immunologic mechanism for which remains undefined, that we term organogenetic tolerance. Induction of organogenetic tolerance for endocrine pancreas is critically dependent on the methodology employed to transplant pig pancreatic primordia.Citation3,Citation10–Citation13,Citation18,Citation19 The phenomenon may be applicable to other organs with appropriate modifications in techniques used for implantation. For example, adaptations in methodology used to xenotransplant embryonic pig kidneysCitation11 so as to result in engraftment that recapitulates the pattern observed following xenotransplantation of embryonic pancreas (i.e., renal cells engrafted in mesenteric lymph nodes) could render hosts tolerant to the embryonic kidney tissue and also to cells originating from adult kidneys from the same donor species. Successful induction of organogenetic tolerance to pig organs other than endocrine pancreas would increase the donor pool and, by virtue of eliminating the need for immune suppression, reduce the risk of transplantation so as to revolutionize organ replacement therapy.
Abbreviations
| E | = | embryonic day |
| ES | = | embryonic stem cell |
| GALT | = | gut associated lymphoid tissues |
| LEW | = | lewis |
| STZ | = | streptozotocin |
Figures and Tables
Figure 1 Intravenous glucose tolerance in rhesus macaques. Glucose in peripheral venous blood was measured prior to intravenous infusion of 0.5 g/kg over 30 seconds as 50% dextrose (Time 0) and at several times after infusion in three fasted rhesus macaques either prior to administration of 60–140 mg/kg intravenous STZ (Pre-STZ); 5 days following administration of STZ (Post STZ); or 3 months following transplantation of 20–40 E28 pig pancreatic primordia in mesentery of STZ-diabetic macaques (Post-TX). Levels measured at 60 minutes after intravenous glucose administration were not different from levels measured at Time 0 in Pre-STZ or Post-TX groups [Bonferroni Multiple Comparisons Test, p < 0.05, two tailed analysis (GraphPad Instat 3, San Diego CA)]. In contrast, levels measured at 60 minutes after intravenous glucose infusion were elevated relative to those measured at Time 0 in the Post-STZ group. Data are shown as mean ± SE (3 macaques).
![Figure 1 Intravenous glucose tolerance in rhesus macaques. Glucose in peripheral venous blood was measured prior to intravenous infusion of 0.5 g/kg over 30 seconds as 50% dextrose (Time 0) and at several times after infusion in three fasted rhesus macaques either prior to administration of 60–140 mg/kg intravenous STZ (Pre-STZ); 5 days following administration of STZ (Post STZ); or 3 months following transplantation of 20–40 E28 pig pancreatic primordia in mesentery of STZ-diabetic macaques (Post-TX). Levels measured at 60 minutes after intravenous glucose administration were not different from levels measured at Time 0 in Pre-STZ or Post-TX groups [Bonferroni Multiple Comparisons Test, p < 0.05, two tailed analysis (GraphPad Instat 3, San Diego CA)]. In contrast, levels measured at 60 minutes after intravenous glucose infusion were elevated relative to those measured at Time 0 in the Post-STZ group. Data are shown as mean ± SE (3 macaques).](/cms/asset/72ba3ca5-d63e-48d7-a1cc-ed5a588cd248/kogg_a_10913283_f0001.gif)
Figure 2 Photograph of kidney from a STZ-treated rat transplanted previously with embryonic pig pancreas in mesentery. The photo was taken 4 weeks after implantation of pig islets in kidney. The whitish well-demarcated graft (white arrow) and the origin of a venous blood vessel (black arrow) are shown. Reproduced with permission from the American Society for Investigative Pathology.Citation12
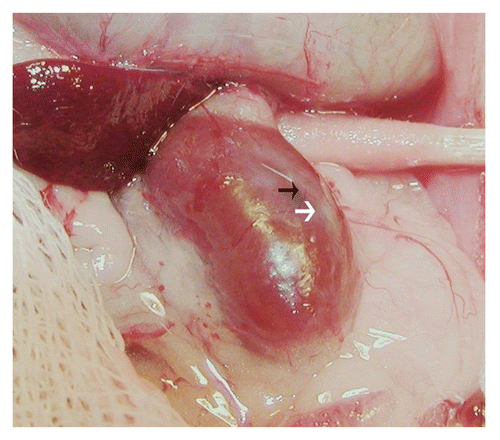
Figure 3 Photomicrographs of kidney from a diabetic rat into which embryonic pig pancreas had been transplanted in mesentery and pig islets had been transplanted subsequently in kidney stained using anti-insulin antibody (A and C) or control antibody (B and D) and sections hybridized to antisense (E) or sense (F) porcine proinsulin mRNA probes. Arrowheads delineate an expanded subcapsular space (A and B). Arrows delineate tissue in the subcapsular space that stains positive for insulin (red-brown) (C) or positive staining for porcine proinsulin mRNA (E). PT, proximal tubule (A and B). Scale bars 80 µm (A and B) and 10 µm (C–F). Reproduced with permission from the American Society for Investigative Pathology.Citation12
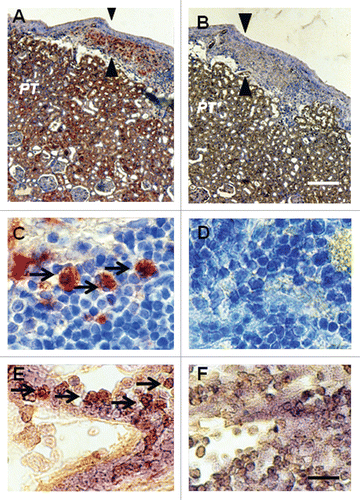
Figure 4 Photomicrographs of the contralateral kidney from a diabetic rat into which embryonic pig pancreas had been transplanted in mesentery and pig islets had been implanted subsequently in the other kidney (A–D) or of a kidney from a diabetic rat in which pig islets had been implanted without prior transplantation of E28 pig pancreatic primordia (E and F) stained using anti-insulin antibody (A, C and E) or control antibody (B, D and F). Arrowheads delineate a normal sized subcapsular space (A and C) or expanded subcapsular space (E). PT, proximal tubule (A, B, E and F). M, medulla (A and B). Scale bars 100 µm (A and B) 10 µm (C and D) or 40 µm (E and F). Reproduced with permission from the American Society for Investigative Pathology.Citation12
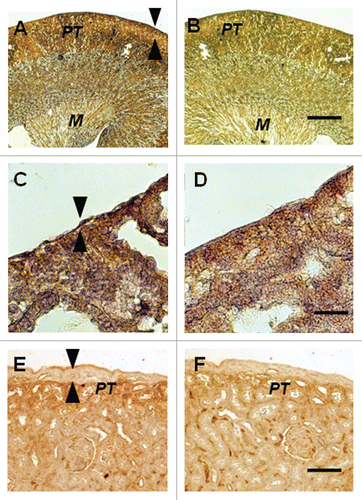
Figure 5 Fluorescence microscopy of tissue sections originating from (A) a normal porcine pancreas or (B–D) a diabetic rat that had been transplanted with embryonic pig pancreas in mesentery and subsequently with porcine islets in kidney: (B) a subcapsular section from kidney, T tubule, RC Renal Capsule; (C) a section of mesenteric lymph node, GC, germinal center, INSET enlargment; and (D) renal cortex, T, tubule. Arrows (A–C) delineate pig X chromosomes. Scale bar 10 um (D). Reproduced with permission from the American Society for Investigative Pathology.Citation12
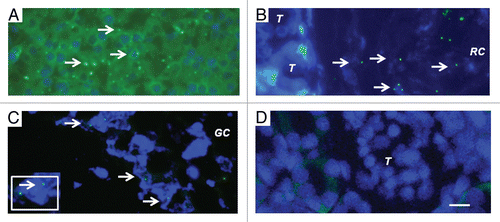
Figure 6 Electron micrographs of rat kidney following sequential transplantation of E28 pig pancreatic primordia in mesentery and implantation of porcine islets in kidney. (A) Subcapsular space. T, renal tubule; Cell containing granules with a crystalline core surrounded by a clear space is delineated by an arrow; Macrophages are delineated by arrowheads. (B) Enlargement of macrophages. (C) Enlargement of granules with a crystalline core surrounded by a clear space. Scale bar 5 um (A). Reproduced with permission from the American Society for Investigative Pathology.Citation12
Acknowledgements
Supported by George M. O'Brien Center DK079333 and grant 1-2008-37 from JDRF.
References
- Hammerman MR. Pancreas and kidney transplantation using embryonic donor organs. Organogenesis 2004; 1:3 - 13
- Hammerman MR. Transplantation of kidney and endocrine pancreas: The window opens. Organogenesis 2007; 3:59 - 66
- Rogers SA, Chen F, Talcott MR, Faulkner C, Thomas JM, Thevis M, et al. Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques. Xenotransplantation 2007; 14:591 - 602
- Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DKC. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation 2006; 13:488 - 499
- Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transplant Immunol 2009; 21:81 - 86
- Groth CG, Korsgren O, Tibell A, Tollemar J, Moller E, Bolinder J, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet 1994; 344:1402 - 1404
- Hering B, Wijkstrom M, Graham M, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006; 12:301 - 303
- Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in non-human primates by targeting costimulation pathways. Nat Med 2006; 12:304 - 306
- van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transpl 2009; 9:2716 - 2726
- Rogers SA, Chen F, Talcott M, Hammerman MR. Islet cell engraftment and control of diabetes in rats following transplantation of pig pancreatic anlagen. Am J Physiol 2004; 286:502 - 509
- Rogers SA, Liapis H, Hammerman MR. Normalization of glucose post-transplantation of pig pancreatic anlagen into non-immunosuppressed diabetic rats depends on obtaining anlagen prior to embryonic day 35. Transplant Immunol 2005; 14:67 - 75
- Rogers SA, Mohanakumar T, Liapis H, Hammerman MR. Engraftment of cells from porcine islets of Langerhans and normalization of glucose tolerance following transplantation of pig pancreatic primordia in non-immune suppressed diabetic rats. Am J Pathol 2010; 177:854 - 864
- Rogers SA, Chen F, Talcott M, Liapis H, Hammerman MR. Glucose tolerance normalization following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic ZDF rats. Transplant Immunol 2006; 16:176 - 184
- Wu GS, Korsgren O, Zhang JG, Song ZS, Van Rooijen N, Tibell A. Pig islet xenograft rejection is markedly delayed in macrophage-depleted mice: a study in streptozotocin diabetic animals. Xenotransplantation 2000; 7:214 - 220
- Wennberg L, Song Z, Benet W, Zhang J, Nava S, Sundberg B, et al. Diabetic rats transplanted with adult porcine islets and immunosuppressed with cyclosporine A mycophenolate mofetil and leflunomide remain normoglycemic for up to 100 days. Transplantation 2001; 71:1024 - 1033
- Korsgren O, Wallgran AC, Satake M, Karlsson-Parra A. Xenograft rejection of fetal porcine islet-like cell clusters in the rat: effect of active and passive immunization. Xenotransplantation 1999; 6:271 - 280
- Schroeder RA, Marroquin CE, Kuo PC. Tolerance and the “Holy Grail” of transplantation. J Surg Res 2003; 111:109 - 119
- Tchorsh-Yutsis D, Hecht G, Aronovich A, Shezen E, Klionsky Y, Rosen C, et al. Pig embryonic pancreatic tissue as a source for transplantation in diabetes: transient treatment with anti-LFA1, anti-CD48 and FTY720 enables long term graft maintenance in mice with only mild ongoing immune suppression. Diabetes 2009; 58:1585 - 1594
- Hecht G, Eventov-Friedman S, Rosen C, Shezen E, Tchorsh D, Aronovich A, et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci USA 2009; 106:8659 - 8664
- Eloy R, Haffen K, Kedinger M, Griener JF. Chick embryo pancreatic transplants reverse experimental diabetes of rats. J Clin Invest 1979; 64:361 - 373
- Abraham EJ, Kodama S, Lin JC, Ubeda M, Faustman DL, Habener JF. Human pancreatic islet-derived progenitor cell engraftment in immunocompetent mice. Am J Pathol 2004; 164:817 - 830
- Rossini AA, Greiner DL, Mordes JP. Induction of immunologic tolerance for transplantation. Physiol Rev 1999; 79:99 - 141
- Korsgren O. Acute cellular xenograft rejection. Xenotransplantation 1997; 4:11 - 19
- Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA 2009; 106:14978 - 14983
- Hammerman MR. Xenotransplantation of pancreatic and kidney primordia: Where do we stand?. Transplant Immunol 2009; 21:93 - 100
- Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med 2006; 203:497 - 500
- Crispe IN. Hepatic cells and liver tolerance. Nat Rev Immunol 2003; 3:51 - 62
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med 2006; 203:519 - 527
- Matsunaga T, Rahman A. In search of the origin of the thymus: the thymus and GALT may be evolutionarily related. Scand J Immunol 2001; 53:1 - 6
- Youson JH, Al-Mahrouki AA. Ontogenetic and phylogenetic development of the endocrine pancreas (islet organ) in fishes. Gen Comp Endocrinol 1999; 116:303 - 335
- Jansen A, Vorbi HAM, Jeucken PHM, Bruning GJ, Hooljkass H, Drexhage HA. An immunohistochemical study on organized lymphoid cell infiltrates in fetal and neonatal pancreases: a comparison with similar infiltrates found in the pancreas of a diabetic infant. Autoimmunity 1993; 15:31 - 38
- Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci USA 2005; 102:17729 - 17733
- Chen Y, Inobe J, Marks R, Gonnela P, Kuchroo VK, Weiner HJ. Peripheral deletion of antigen reactive T cells in oral tolerance. Nature 1995; 376:177 - 180
- Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, et al. Mesenteric lymph nodes are critical for the induction of high dose oral tolerance in the absence of Peyer's Patches. Eur J Immunol 2002; 32:1109 - 1113
- Mandel TE. Fetal islet xenotransplantation in rodents and primates. J Mol Med 1999; 77:155 - 160