Abstract
Xenotransplantation was proposed a long time ago as a possible solution to the world-wide shortage of human organs. For years, researchers in this field have almost exclusively directed their efforts towards combating the immunological barrier that precluded the long-term xenograft survival. Studies have been conducted in both small and large animal models and the most relevant results have been obtained in pre-clincal studies, specifically those utilising the pig-to-nonhuman primate combination. In this context, a better understanding of the immunological mechanisms underlying the rejection of a xenograft have allowed the identification of specific targets of intervention that have resulted in considerable improvements in survival of porcine organs or cells in nonhuman primates. However it has also become apparent that if xenotransplantation has to enter the clinical arena, a multidisciplinary approach will be needed to comprehensively tackle the different issues related to the use of a xenograft to cure human disease.
In this regard, the safety, ethics and regulatory aspects of xenotransplantation are currently being aggressively addressed to enable the initiation of xenotransplantation with a favourable risk/benefit ratio.
Introduction
Xenotransplantation, more commonly defined as the transplantation of organs, tissues or cells from one species to another, has previously been proposed as a possible solution to the world-wide shortage of human organs for patients in terminal organ failure. Whilst considerable progress has been achieved in this field in the last few years, a few issues still need to be satisfactorily addressed to enable xenotransplantation to become a real therapeutic option for our patients.
Considerable advances in this exciting but difficult field took place in the eighties and nineties, both in the European Union and outside Europe. These findings provided important information to enable better understanding of the xenograft rejection process and gain insight into safety aspects relating to xenotransplantation.
During the same period, however, emerging technologies such as regenerative medicine and stem cell research attracted much attention and support from funding agencies, as these approaches were viewed as more promising for patients in organ failure. This resulted in a lack of public and private funding to support xenotransplantation research in many laboratories worldwide, ultimately forcing many research entities to fold their xenotransplant activities and redirect their science towards these approaches with a seemingly higher potential for success.
Whilst it is undeniable that research into stem cells and regenerative medicine has resulted in the generation of some significant results, it has also become apparent that the biological processes involved in such scientific disciplines are much more complex than originally anticipated and will not be immediately offered to those in need. As a consequence of this slower than expected progress, research efforts in xenotransplantation have resumed in many countries world wide, including the United States, the European Union, Australia, Japan and China.
The Advantages of Xenotransplantation and the Use of the Pig as a Potential Source of Organs or Cells
The realisation of xenotransplantation as a clinical reality would result in numerous advantages to the transplant patient (). Xenotransplantation would provide an unlimited supply of organs of any type or size for human transplantation.Citation1 The availability of such organs would minimise the time spent on the waiting list, avoid progressive clinical deterioration which usually occurs while waiting for an organ and allow elective and programmed surgery in ideal clinical conditions. It would also allow the large number of patients, for whom organs are not currently available, to be transplanted. The reduced time spent on the waiting list would considerably reduce the costs related to the treatment of patients with terminal organ failure, such as those on dialysis. Moreover, xenotransplantation would enable better organisation of the activities of transplant units. Ischaemia time, which is considered an important factor for the long-term survival of an allograft, would be reduced substantially. Finally, the widespread availability of organs would hopefully eliminate the repeatedly denounced illegal trading of human organs.Citation2
Furthermore, xenotransplantation offers an additional, unique advantage over allotransplantation. Indeed, nowadays only minor interventions are possible in deceased donors to improve the health status of a donor organ prior to transplantation and enhance its acceptance by the recipient. Indeed, deceased organ donors are most often in unstable conditions that would preclude manoeuvres to improve the outcome of the transplanted organ. In contrast, xenotransplantation offers the tremendous advantage of donor organ modification to render the graft more “compatible” with human recipients, ahead of time of the actual use of the organ.
The pig is currently viewed as the most appropriate source of organs for the hypothetical clinical application of xenotransplantation. Indeed, several advantages have been associated with the use of this species (). First, the pig has many anatomical and physiological similarities with man.Citation3 Earlier studies have demonstrated that porcine organs may reach the same size and have the same degree of efficiency as their human counterparts.Citation1 Furthermore, as basic metabolic processes are shared by all mammals, most metabolic activities, such as pH and osmolarity, will not be an obstacle to xenotransplantation in primates using porcine organs.Citation4 Second, the pig has a short gestation period (around 115 days) and produces large litters of 10 or more offspring, that rapidly grow to the size necessary for xenografting into human adults. Third, pigs can easily be bred under qualified pathogen-free conditionCitation5 such that the majority of known zoonotic agents can be eliminated. Fourth, this species is widely used for food, and therefore faces fewer ethical objections than the use of nonhuman primates. In this respect, it is worth mentioning that more than 80 million pigs are reared and slaughtered each year in the United States to provide meat and other products.Citation1 For these considerations, a consensus has grown within the scientific community working in this field, that the pig represents the preferred donor species for future clinical xenotransplantation.
Xenotransplantation: The Need for an Integrated, Multidisciplinary Approach to Tackle Currently Outstanding Issues
Xenotransplantation research undertaken in the nineties has clearly taught us that success in this field is dependent on a vast array of knowledge that goes far beyond understanding and addressing the rejection process. Certainly, the immunological barrier has historically constituted a major obstacle to the long-term survival of pig xenografts transplanted into primates and still needs to be appropriately overcome in order to allow clinical studies. However, other issues, previously unknown or possibly underestimated, have now arisen. These include the need to broaden knowledge regarding biosafety aspects of this practice, to deepen understanding of the physiological compatibility between pigs and primates and clearly define the Ethical and Regulatory requirements that will need to be adequately addressed and satisfied prior to allowing the clinical application of xenotransplantation.
In this context, it is beyond any doubt that, at this stage, advancements in xenotransplantation that will allow the clinical application and success of this field will be entirely dependent on a multidisciplinary approach that will integrate very diverse skills. In this light, the European Union has recently promoted a xenotransplantation research programme across Europe entitled “Xenome”. Xenome is one of the EU funded “Integrated Projects” spread over up to 5 years. The project, launched at the end of 2006, is a research effort that includes 22 academic/private institutions in 11 countries with a fairly balanced representation and distribution of funding between academic and private entities within EU member states. The key areas of research pursued in Xenome include immunology, safety, genetic engineering and physiology, as well as ethics, and social and regulatory aspects of xenotransplantation. In addition, in accordance with the mission of any Integrated Project, effort has been directed towards the dissemination of knowledge and the training of new generations of investigators in Europe. For financial and ethical reasons, all the in vivo studies in Xenome are confined to a limited number of carefully planned preclinical xenotransplantation studies in the primate.
The Immunological Barrier
The immunological response to xenografts remains a central issue that will need to be dealt with satisfactorily if clinical xenotransplantation trials are to be initiated. In particular, a complete understanding of the immunological responses leading to xenograft rejection has yet to be fully acquired. Nonetheless, significant advances have been achieved in the understanding of such processes and at least five approaches are being pursued to overcome the immune obstacles to the long-term survival of a porcine xenograft in a primate (). The first approach entails the development of user-friendly pharmacological immunosuppression, finely adapted to specifically counteract the immunological events underlying the xenograft rejection process.Citation6,Citation7 Secondly, much research has been directed towards the development of tolerance inducing regimens, i.e., the development of strategies that would allow long-term survival of porcine xenografts in the absence of immunosuppression.Citation8–Citation10 The establishment of accommodation, defined as long-term graft survival, notwithstanding the continuing presence of xenoreactive antibodies and complement,Citation11 is regarded as a third strategy which may enable long-term graft survival. Furthermore, excellent results have recently been achieved by utilising encapsulation as a strategy to protect cellular xenografts from immune damage, whilst allowing optimal metabolic function of the transplanted cell mass.Citation12 Finally, the last approach designed to tackle the rejection process is that obtained via genetic engineering of the donor source, to render the xenograft more immunologically compatible with man.Citation13
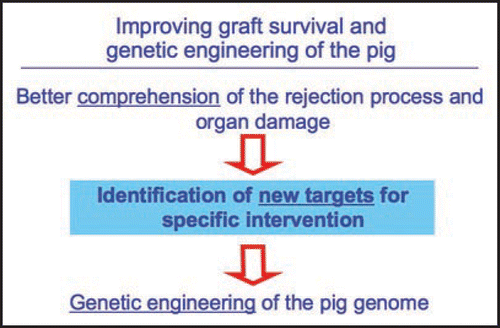
In any case, as for the development of any novel strategy to cure human diseases, the success of xenotransplantation is intimately linked to the fine comprehension of the mechanisms underlying the pathological insult, the rejection process in our case, allowing the identification of novel and specific targets for interventions. In the case of xenotransplantation, this may ultimately translate into the specific engineering of the pig genome to introduce (or subtract) traits that will enhance graft survival ().
As the rejection process differs considerably between solid organ and cellular xenotransplantation, these two types of xenografts will be reviewed separately, focussing specifically on the context of pig-to-nonhuman primate xenografts.
Solid organ xenotransplantation.
Our understanding of the immunological processes which occur following pig-to-nonhuman primate xenotransplantation has grown considerably in the last few years. With specific regard to solid organ xenotransplantation, the mechanisms of hyperacute rejection (HAR) and acute humoral xenograft rejection (AHXR) [also called acute vascular rejection (AVR)Citation14 or delayed xenograft rejection (DXR)Citation15], the two key immunological hurdles affecting the long-term survival of solid pig organs in the primate, have now been fairly well elucidated.
Until recently, HAR was the primary immunological barrier to the xenotransplantation of pig organs in the primate. HAR is characterised by diffuse interstitial haemorrhage, oedema and thrombosis of small vessels and capillaries.Citation16 These changes are secondary to endothelial cell activation and damage caused by pre-existing anti-pig antibodies. Clarification of the mechanisms responsible for the onset of HAR has demonstrated the central role of complement and antibodies in its pathogenesis,Citation17 allowing the development of approaches which interfere with either the interaction of xenoreactive natural antibodies with their primary target, Galα1-3Galβ1-4GlcNAc-R structures (known as αGal epitopes)Citation18,Citation19 or, alternatively, with the complement cascade.
In this context, the emphasis of research has been on the production of genetically engineered pigs expressing inhibitors of the human complement cascade such as human decay accelerating factor (hDAF; CD55),Citation13,Citation20 membrane cofactor protein (MCP; CD46,Citation21) or CD59.Citation22 The validity of this approach has now been convincingly demonstrated by several groupsCitation20,Citation23,Citation24 who have shown that similarly engineered pig organs usually do not undergo HAR once transplanted into primates.
However, xenografts which survive HAR almost inevitably fail eventually as a consequence of AHXR. Several elements have been implicated in the pathogenesis of AHXR. Its pathology is primarily characterised by vascular thrombosis, blood extravasation and oedema.Citation16 Deposits of fibrin, immunoglobulins and complement in the graft do not differ substantially from those observed in HAR. Cellular infiltrates include neutrophils, macrophages, CD8+ T cells and few NK cells.Citation16 Recently, however, with the use of xenografts deriving from αGalT−/− pigs and the introduction of novel immunosuppressive strategies, the pattern of AHXR is changing towards a picture compatible with a prominent thrombotic microangiopathy.Citation25 As for HAR, the identification of specific targets of intervention for AHXR should allow scientists to overcome this immune barrier, ultimately further extending xenograft survival. To this end, at least three different approaches are currently being explored. First, ongoing research regarding the role of complement in AHXR has demonstrated that complement deposition is consistently observed in the majority of xenografts rejected due to AHXR and is considered a hallmark of this process.Citation23 In this light, interventions have been designed to completely prevent complement activation that is only partially abrogated when using organs from the existing pig lines transgenic for human complement regulators.Citation26 Indeed, whilst it is clear that such lines can dramatically reduce complement-mediated damage and prevent HAR, it is also certain that such complement control is incomplete, possibly as a consequence of the overwhelming complement activation that at some stage supersedes the capacity of transgenically expressed proteins to control complement activation. Secondly, efforts are underway to reduce the immunogenicity of transplanted organs. To this end, several laboratories have recently obtained pig lines lacking the galactosyltransferase gene (αGalT−/−). These animals do not express αGal epitopes in the majority of cases, although the reported presence of low residual levels of αGal epitopes in αGalT−/− pigs, possibly added to lipids by iGb3 synthase, have been described by Sharma and colleagues.Citation27 However, despite the absence of αGal residues, AHXR is still observed following the transplantation of αGalT−/− organs and in many cases, it has been demonstrated that AHXR of αGalT−/− organs is associated with the presence of circulating elicited anti-non-αGal antibodies.Citation28 Nonetheless, a direct causative role of such non-αGal antibodies has not yet been proven and additional studies to further investigate these observations are eagerly awaited. Engineering of pig lines that are deficient in one or more of the target molecules recognised by anti-non-αGal antibodies may indeed be necessary to improve current survival rates. Finally, molecular incompatibilities between the coagulation systems of the pig and primate have previously been reported.Citation29 These are currently being studied as possible additional targets of intervention to overcome frequently observed coagulopathy and fibrin deposition in xenografts explanted in the presence of AHXR.
In addition to HAR and AHXR, a third category of acute rejection, termed acute cellular xenograft rejection (ACXR) has also been described. Notwithstanding in vitro demonstrations of strong cellular responses,Citation30 and in contrast to in vivo results in the hamster-to-rat model,Citation31 it is important to underline that ACXR per se, does not lead to solid organ failure following pig-to-primate organ xenotransplantation. Therefore, at this stage, it would seem that graft damage directly mediated by the cell-mediated immune response can be largely prevented by the immunosuppressive regimens currently available.Citation30 On the other hand, a contribution of T cells to the development of the elicited anti-xenograft humoral immune response cannot be ruled out.
With regards to the phenomenon of chronic rejection, very little information is available in the pig-to-primate context. This is most likely due to the fact that long-term survival of xenografts is not routinely obtained due to the problems posed by AHXR. However, recently, a phenomenon described as chronic xenograft vasculopathy was reported in a pig-to-baboon heterotopic cardiac transplantation model, despite chronic immunosuppression, in primate recipients of αGalT−/− hearts.Citation32 In these animals, both humoral and cellular features of rejection were associated with the development of chronic xenograft vasculopathy, characterised by intimal thickening, fibrin exudation, complement and immunoglobulin deposition and cellular infiltration. This preliminary data warrants further investigation to determine if chronic rejection will impact significantly on long-term xenograft rejection as observed in allotransplantation.
Cellular xenotransplantation.
Pig islets represent the most studied model of cell xenotransplantation in the primate and will be here briefly discussed. In comparison to solid organs, islets have a particular advantage when it comes to rejection. Indeed, islets are not immediately vascularised and express low to negligible levels of the αGal epitope, thereby making them intrinsically resistant to the majority of the natural antibody repertoire.Citation33 Moreover, the isolation and pre-transplant culture procedures result in the loss of most of the islet vasculature, essentially eliminating those few porcine islet vascular endothelial cells which do express αGal epitopes and could otherwise present a target for the xenogeneic humoral response. Therefore, islets are largely resistant to the process of HAR. Nonetheless, islets are not resistant to rejection. Essentially, two immunological barriers have been demonstrated to be the most important in islet xenograft rejection. The first of these has been termed the Instant Blood-mediated Inflammatory Reaction (IBMIR), and occurs immediately following the contact of xenogeneic islets with human blood. Indeed, the exposure of porcine islets to whole blood results in an inflammatory reaction, characterised by macroscopic coagulation, the activation of complement, consumption of platelets and infiltration of leukocytes.Citation34 Although the reaction occurs with a kinetics similar to HAR, failure to observe antibody deposition on the graft is a distinguishing feature.
The onset of the IBMIR may account for the premature loss of grafted islets following intraportal islet infusion and the consequent large tissue volume required to achieve a functional islet mass following transplantation via this route. Whilst heparin treatment is standard procedure for intraportal islet transplantation, the use of novel approaches such as dextran sulfate,Citation35 or surface-coating islets with heparinCitation36 may prove to be beneficial in further abrogating this response.
In the absence of immunosuppression, islets which are not destroyed by the IBMIR, primarily undergo cellular rejection mediated in large part by T cells and macrophages.Citation37,Citation38 Following the initial recruitment of CD4+ T cells and macrophages to the graft, the secretion of a cascade of chemokines leads to a massive influx of macrophages and helper T cells, followed by the infiltration of eosinophils, CD8+ T cells and neutrophils, ultimately resulting in graft destruction.Citation39
Islet Xenotransplantation as the Most Advanced Model of Pig-to-Primate Xenotransplantation
As islet rejection mechanisms involve a primarily cellular immune response, the principal immunosuppressive strategies applied have preferentially targeted this rejection process. Recently, three independent research groups have obtained porcine islet xenograft survival for 6 months in nonhuman primate transplantation models via the development of novel immunosuppression or encapsulation strategies.Citation40–Citation42 These studies will be briefly discussed below. The first strategy entailed the use of multi-agent immunosuppression regimens, principally focused on the prevention of T-cell-dependent rejection by the impairment of costimulatory pathways. Interestingly, using such an approach, sustained normoglycemia in diabetic nonhuman primates was achieved for more than 6-months by two different groups with immunosuppressive therapy which included anti-T cell agents interfering with the CD154/CD40 pathway. In particular, Hering and colleagues transplanted adult porcine islets into streptozotocin-diabetic cynomolgus macaques applying an immunosuppression regimen consisting of anti-IL2R and anti-CD154 monoclonal antibodies, FTY720, everolimus and leflunomide.Citation40 Porcine islets functioned for over 180 days and a 100% xenograft survival rate was observed at day 100 after transplant.
Using a similar multi-agent immunosuppression approach, Cardona and colleagues transplanted neonatal porcine islets to pancreatectomized rhesus macaques with an immunosuppression regimen consisting of anti-IL2R and anti-CD154 monoclonal antibodies, sirolimus and belatacept (a high-affinity derivative of CTLA4-Ig).Citation41 In this study, sustained insulin independence was achieved with a median survival time greater than 170 days, with the longest surviving graft having a duration of 345 days.Citation43
Despite these pre-clinical successes, the complex immunosuppression protocols and the reported association of anti-CD154 antibodies with thromboembolic events,Citation44 make these immunosuppression regimens clinically inapplicable. For this reason, the latter group is currently studying the efficacy of an alternative multi-drug immunosuppressive regimen, with a chimeric anti-CD40 antibody replacing the prothrombotic anti-CD154 antibody for the engraftment of neonatal porcine islets in nonhuman primates.Citation43 Encouraging ongoing experiments indicate that this CD28/CD40 costimulatory blockade provides adequate initial xenograft function with improved glucose control, suggesting that with further refinements, this immunosuppressive therapy might become a valid alternative to anti-CD154 based immunosuppressive regimens.
The third group applied an immunosuppression-free approach to protect transplanted islets from the cell-mediated immune response. Whilst successful transplantation of encapsulated porcine islets was described by Sun and colleagues in 1996,Citation45 these results have proven difficult to reproduce. Using a novel approach to encapsulation, outstanding results were recently obtained by Gianello and colleagues with alginate encapsulated adult pig islets. As a first step, a proof of concept study demonstrated the stability and biocompatibility of alginate-encapsulated pig islets and indicated that xenografts can survive for up to 6 months in non-diabetic nonhuman primates, maintaining the ability to respond to an in vitro glucose challenge.Citation42 More recently, this group has been able to achieve sustained normoglycemia following the subcutaneous transplantation of a monolayer cellular device composed of alginate in four diabetic nonhuman primates. In the complete absence of immunosuppression, normoglycemia was maintained in these recipients for up to 6 months. These impressive preliminary results which await confirmation, indicate that the device developed can control the diabetic status for up to 6 months without any immunosuppression, an important feature which will be of critical significance in a possible future application of such technology in diabetic patients.Citation12
It is of interest that these excellent results obtained in the islet transplantation field have utilised unmodified pigs (both neonatal and adult). In this light, it is not unreasonable to expect that islet xenotransplantation will further benefit from genetic modification of the donor pig. As a consequence of the inherently low to negligible expression of αGal on pig islets, the αGalT−/− pigs may not have a great impact on islet xenotransplantation. In contrast, the use of transgenic pigs possessing high-level expression of antithrombotic molecules could lead to considerable protective effects, mitigating the IBMIR and allowing further islet resistance following exposure to the bloodstream. In this regard, encouraging results have been obtained with CD39 transgenic mice showing a delay in clotting time when CD39 transgenic islets were exposed to human blood.Citation46 Similarly, the use of transgenic pigs expressing the NK and T-cell apoptotic inducer TRAILCitation47 as islet sources, may also prove beneficial to islet xenotransplantation.
Xenotransplantation and Tolerance
A number of approaches have been applied in efforts to induce tolerance in xenotransplant models. These include mixed hematopoietic chimerism, costimulatory blockade, donor-specific transfusion and thymic transplantation.Citation48,Citation49 Whilst tolerance has been achieved in xenotransplant models utilising rodent recipients, achieving complete tolerance in large animal models is still elusive. Furthermore, achieving xenogenic tolerance appears to be a much more difficult process than that required for allografts, most likely due to the significant role of the humoral response in all phases of xenograft rejection. In this light, the effective establishment of xenograft tolerance in large animals will require approaches to tackle both B and T cell-mediated xenoimmune responses.Citation50
B-cell tolerance to xenografts has been achieved in rodents following bone-marrow transplantation to induce B-cell anergy and deletion, preventing the generation of newly-formed and pre-existing antibody responses towards xenoantigens.Citation51 Additional strategies to induce B-cell xenograft tolerance have included gene-therapy and treatment with leflunomide (reviewed in ref. Citation52). However, the effects of such procedures in nonhuman primate responses have yet to be evaluated.
T-cell tolerance inducing strategies have been extensively assessed in rodent xenograft models, however, few have shown efficacy in large animal models. These strategies include co-stimulatory blockade, thymic transplantation and hematopoietic chimerism. Co-stimulation blockade therapy aims to render T-cells unresponsive to antigenic stimulation by inhibiting necessary co-stimulator molecule engagement during binding of the T-cell receptor with antigen. Whilst both the CD80/86-CD28 and CD40-CD40L pathways have been successfully targeted in rodent xenotransplant models, providing markedly prolonged xenograft survival, tolerance using these agents has not been achieved in pig-to-primate models. Nonetheless, the inclusion of these agents in immunosuppressive protocols has resulted in significant enhancements in graft survival in xenograft recipients.Citation32,Citation40,Citation41,Citation53
Prolonged survival of xenografts has been reported following co-transplantation of porcine kidneys with donor thymic tissue. Studies in rodent models have demonstrated the ability of the porcine thymus to generate donor-specific tolerance of both rodent and human T-cells towards the pig.Citation54–Citation56 Using this approach, Yamada et al. were able to extend the survival of pig kidney xenografts in the nonhuman primate, demonstrating sustained normal graft function in the absence of rejection for up to 83 days.Citation53 In this study, the authors were able to demonstrate pig-specific unresponsiveness via mixed lymphocyte reaction in two long-term surviving recipients, and the absence of a pig-specific cytotoxic T lymphocyte response in one case. However, the role of an immune-mediated mechanism behind the mild and focal thrombotic microangiopathy observed in these grafts has yet to be ruled out. Furthermore, the requirement for continuous administration of a human anti-CD154 monoclonal antibody, occasionally associated with thrombotic complications, suggests that further refinements will be necessary before complete tolerance can be achieved.
Finally, the ability of mixed hematopoietic chimerism to generate xenograft tolerance has been successfully demonstrated in pig-to-mouse models.Citation9,Citation57,Citation58 However, a recipient conditioning regimen able to induce successful stable engraftment of porcine bone marrow in the primate has yet to be identified. This is largely due to the inability to achieve complete T-cell depletion in primates and the need to overcome hematopoietic cell rejection by natural antibodies, NK cells and macrophages.Citation52 However, as transient chimerism has proven sufficient for the establishment of T-cell tolerance to allografts in large animals,Citation49,Citation59 it has yet to be established whether tolerance may indeed be induced in the pig-to-primate combination following the application of such a procedure.
Physiology
Future clinical applications of xenotransplantation can only be envisaged if adequate compatibility between pigs and primates can be demonstrated. Whilst physiological incompatibilities between these two species have been reported,Citation29,Citation60,Citation61 these do not appear to represent an insurmountable challenge to the long-term survival of porcine renal, cardiac xenografts or islet xenografts. Reported incompatibilities include molecular differences between the coagulation system of the pig and primate.Citation29 Indeed, porcine von Willebrand Factor (vWF) has been shown to interact with human platelet receptors with high affinity. Porcine vWF is able to activate quiescent platelets, resulting in platelet aggregation in the absence of shear stress, possibly resulting in elevated pro-coagulant activity.Citation62 Porcine tissue factor pathway inhibitor (TFPI) is not able to neutralize human factor Xa, and is therefore unable to inhibit the direct activation of human prothrombin to thrombin.Citation63,Citation64 In addition, although porcine thrombomodulin has been shown to bind human thrombin and Protein C, the human thrombin-porcine thrombomodulin complex is a poor activator of Protein C. As a consequence, the insufficient production of activated Protein C contributes to enhanced levels of thrombin, favouring the initiation of clotting.Citation64,Citation65
In order to combat such incompatibilities, transgenic modulation of the clotting cascade by de novo expression or induction of anticoagulants, or the elimination of pro-coagulant molecules on the xenogenic vascular endothelium, may represent a potential therapeutic strategy. In this context, several target gene candidates for transgenic expression (e.g., CD39, TFPI, thrombomodulin, hirudin, CD73), or knock-out (e.g., Tissue Factor, PAR3, PAR4, Fgl-2) in pig tissues have been identified. Encouraging results, although only obtained in vitroCitation66 and in small animal models,Citation67–Citation71 have provided a basis for the future genetic manipulation of porcine organs possibly able to overcome thrombotic events that compromise xenograft survival.
Notwithstanding the physiological differences identified, in vivo studies in nonhuman primates suggest that porcine hearts, kidneys and islets are able to function in primates and sustain their life for up to several months.Citation6,Citation32,Citation72 During this time, the organs support normal levels of activity, with the recipients exhibiting normal social behaviour. Together, these in vivo observations suggest that, to date, no insurmountable physiological incompatibility has been identified between the pig and primate.
Safety Aspects
Transplantation per se carries the intrinsic risk of transmitting donor-derived infectious agents to the recipients. This is irrespective of whether donor and recipient are from the same species or not. Indeed, even in the case of clinical allotransplantation, we are still unable to guarantee protection of human recipients from existing donor-derived pathogens. This was clearly demonstrated earlier this year when a newly identified Arena virus was found to be responsible for a cluster of transplant-associated deaths.Citation73
As far as the safety aspects related to xenotransplantation are concerned, considerable progress has been achieved.Citation74 It is beyond any doubt that the exclusion of known exogenous pathogens represents a necessary step toward clinical xenotransplantation. This can be achieved by direct caesarian derivation of founder animals, followed by barrier rearing and regular screening of sentinel animals for the presence of specific pathogens. Whilst such an approach can, in theory, eliminate all known exogenous pathogens from the breeding colony, such an approach cannot eliminate agents in a latent or intracellular state such as porcine endogenous retrovirus (PERV), circoviruses and other agents, or as yet unidentified pathogens.
It is nonetheless encouraging that PERV have not been associated with any current, past or latent infection in xenografted patientsCitation75 and that PERV are susceptible to some of the currently available antiviral agents.Citation76 Furthermore, pig lines have recently been identified which are incapable of transmitting PERV to human cells in vitro.Citation77,Citation78 In addition, genetic manipulation of the porcine genome may provide an additional strategy to remove the viral risk. In this regard both specific knock outs of endogenous retrovirusesCitation79 or the use of short interfering RNAs specific for PERV sequencesCitation80 have been proposed and preliminary data in engineered pigs are very encouraging.Citation81
From a diagnostic standpoint, the development of microarray-based technology capable of rapidly identifying known and as yet unidentified potential infectious agentsCitation82 will allow their timely identification and control in the xenotransplantation setting. In this regard, it is expected that advances in this field will significantly contribute to enhance the safety profile of xenotransplantation.
Regulatory and Ethical Frameworks
To date, a single legal document regulating clinical xenotransplantation procedures world-wide has yet to be developed. Still, several well-written documents have been released in the last few years that should constitute the basis for an international regulatory framework within which clinical xenotransplantation should take place. These documents include the U.S. Public Health Service (PHS) Guideline on Infectious Disease Issues in Xenotransplantation (January 19, 2001),Citation83 the Guidance for Industry—Source Animal, Product, Preclinical and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans—FDA April 2003,Citation84 the Recommendation Rec (2003)10 of the Committee of Ministers to EU member states on xenotransplantationCitation85 and the document prepared following the Xenotransplantation Advisory Consultation held in Geneva in April 2005.Citation86 The aspects of xenotransplantation addressed by these documents are diverse and include efficacy and safety aspects, ethical principles and regulatory frameworks for xenotransplantation. In particular, it comes across very clearly that the initiation of clinical trials must not be exclusively based on the generation of efficacy data in preclinical models. Indeed, beside the demonstration that porcine xenografts are not rejected for a significant period of time using a clinically applicable immunosuppression strategy and meet the physiological requirements of the nonhuman primate recipient, no infectious agents should be identified in porcine xenograft recipients.
Several ethical requirements common to all clinical trials and some specific to xenotransplantation should clearly be satisfied in order to initiate and conduct clinical xenotransplantation studies. The ethical principles which need to be met have recently been outlined by the Ethics Committee of the IXACitation87,Citation88 and encompass ethical considerations for both the human recipient and the donor source animal. As far as the human recipient is concerned, it should be underlined that minors should be denied participation in such trials, and an appropriate informed consent should be prepared that clearly outlines all the caveats and restrictions that could be applied to xenograft recipients involved in initial clinical studies. Furthermore, with regard to source animals, these should be bred in barriered facilities in accordance with the highest standards of animal husbandry, respecting the requirements of the species.
With respect to the preparation of an internationally accepted and applied regulatory framework, a report which formed the basis of the World Health Assembly Resolution WHA57.18 adopted in May 2004 represents a first important step towards the harmonization of xenotransplantation procedures and policies.Citation89 This resolution urged Member States to “allow xenotransplantation only when effective national regulatory control and surveillance mechanisms overseen by National Health Authorities are in place.”
More recently, the Xenotransplantation Advisory Consultation held in Geneva in April 2005, released a statement where the role of each Member State was more comprehensively defined. In particular, the role of Member States in the implementation of such a Resolution could be summarized as follows:
Undertake an inventory of xenotransplantation practices in their country. | |||||
Allow xenotransplantation only if there is an effective regulatory system in place. | |||||
Ensure that the regulatory authorities properly weigh the risks and potential benefits of any clinical trials or procedures before giving authorization. | |||||
Ensure that first class regulatory standards are in place with regards to source animals; authorization of procedures, ethical approval and consent procedures; education of patients, intimate contacts and health-care workers, including those in public health; quality management and auditing of outcomes. | |||||
Ensure that there are effective surveillance systems in place that would identify and manage events that pose a potential danger to public health. | |||||
Ensure transparency about xenotransplantation activities. | |||||
Promote public awareness. | |||||
Taken together, these considerations indicate that all the principles are being put in place to resume clinical xenotransplantation efforts, when appropriate, under the best auspices for its success and minimizing any risks of failure.
Conclusion
The data discussed above clearly indicate that considerable progress has been achieved in this field in the last few years. This primarily includes advancements from immunological, safety and physiological points of view. However, it is the authors opinion and that of many, that it is only when a favourable risk/benefit ratio can be clearly demonstrated in convincing and relevant pre-clinical studies that well designed clinical xenotransplantation trials should be initiated in countries where an appropriate ethical and regulatory framework is in place.
In your genetic engineering approaches, it would appear that the most efficient way to produce a pig that would be a good organ donor for humans would be to make a multi-transgenic animal that has genes targeting different beneficial biological aspects for graft survival. What is the experience or technical status of that in pigs?
Indeed, successful xenotransplantation will require an engineered source animal with multiple genetic modifications. Specific modifications will ultimately result in a better control of the immune response and of the coagulation cascade, and result in a better safety profile of the potential donor pig. At this stage, we certainly have multi-transgenic pigs that co-express, for instance, inhibitors of the coagulation cascade and inhibitors of complement.Citation90 Furthermore, complement regulators have been added to the αGalT−/− background that renders the donor more immunologically “compatible” with man. Pigs with knocked-down PERV expression by PERV-specific shRNA have also been reported.Citation81
Basically, we are independently tackling all the different aspects that need to be addressed to allow long-term and safe survival of xenotransplanted organs. What we need to do now is bring the different traits together in a single donor animal. This will be achieved using cloning technologies and conventional breeding strategies to ensure fertility of the newly generated line.Citation91 Alternatively, sperm-mediated gene transfer could represent another tool.Citation92
I would like to hear more about your experience with cell-based therapies for neurodegenerative diseases such as Parkinson's. The relevance of this technology is often questioned, because in order for it to work, neuronal axons must find their targets, and re-establish functional synapses that will remain stable throughout life. What do you see in your models?
These long-lasting experiments are underway. These experiments can be grossly divided into 2 groups. In the first set of experiments, we will only verify whether genetically engineered neuroblasts obtained from CTLA4-Ig transgenic pigsCitation93 can survive in the brain of immunosuppressed nonhuman primates. These studies will also have to verify whether surviving xenografts are able to establish appropriate synaptic connections with the relevant recipient parenchyma. In the second set of experiments, we will verify whether graft survival can be associated with graft function, resulting in some sort of functional benefit. These studies will include neuroradiological investigations (PET-scan imaging with F-Dopa) and motor tests.
Can you develop a transgenic pig with organs that are less susceptible to ischemia- reperfusion injury?
We do not plan to undertake this in the context of XENOME, but it is obviously a very important issue and we will perform assessments of ischemia/reperfusion injury in the context of our xenotransplantation experiments.
Does XENOME have a data safety monitoring mechanism to verify pre-clinical results prior to proceeding into human trials? Also does XENOME require that results be verified by another laboratory prior to proceeding with clinical trials? The reason I ask is that you have described successful xenotransplantation of islets in non-immunosuppressed hosts using alginate encapsulation. However, the history of this approach is that it is not uniformly successful.
XENOME has no plan to proceed with clinical trials but only to get xenotransplantation closer to its clinical application. To this end, a wide range of experiments in the most diverse fields have been planned, which involve a multidisciplinary team of scientists, experts in ethics and experts in law. Furthermore, there will be extensive interaction with the public to make sure that all potential stakeholders are involved, bringing them up to speed with the advancements in this field. In all cases, as already stated, the initiation of clinical trials will only be possible when a favourable risk/benefit ratio exists, and in the context of an appropriate ethical and regulatory framework. With regard to the results generated by Gianello and colleagues,Citation12 we are all intrigued by the very promising results of their latest alginate experiments. We still do not know why they are substantially different from previous studies using alginate technology. However, it is my understanding that with this new approach, oxygen diffusion is improved, and pore size or molecular weight cut-off is more efficient which somehow leads to such significant results.
It seems to me that XENOME should require that results have to be concurrent in another laboratory before proceeding into a clinical trial.
We have not formally specified independent replication. However, in principle we could accommodate such a requirement as the program utilises two nonhuman primate species. If a strategy is successful in one species, for instance in cynomolgus monkeys, the concept could be demonstrated in the other species available to the network (baboon). We already have this internal arrangement but the important thing to understand is that XENOME does not have the intention to go into man.
Figures and Tables
Table 1 The advantages of xenotransplantation
Table 2 The advantages of the pig as a source of organs for clinical xenotransplantation
Table 3 Strategies to extend xenograft survival
Acknowledgements
This work was supported by CORIT (Consorzio per la Ricerca sul Trapianto d'Organi, Padua, Italy), the Italian Ministry of Health, the Veneto Region and the EU FP6 Integrated Project “Xenome”, contract # LSHB-CT-2006-037377.
References
- Cooper DK, Ye Y, Rolf JLL, Zuhdi N. Cooper DK, Kemp E, Reemtsma K, White DJ. The pig as potential organ donor for man. Xeno-transplantation. The transplantation of organs and tissues between species. 1991; 1st Springer-Verlag 481 - 500
- Abboud O, Abbud-Filho M, Abdramanov K, Abdulla S, Abraham G, Abueva A, et al. The Declaration of Istanbul on organ trafficking and transplant tourism. Kidney international 2008; 74:854 - 859
- Hammer C. Physiological obstacles after xenotransplantation. Annals of the New York Academy of Sciences 1998; 862:19 - 27
- Hammer C. Xenotransplantation: facts and fiction. Annales chirurgiae et gynaecologiae 1997; 86:195 - 201
- Iverson WO, Talbot T. Definition of a production specification for xenotransplantation. A European perspective. Annals of the New York Academy of Sciences 1998; 862:121 - 124
- Baldan N, Rigotti P, Calabrese F, Cadrobbi R, Dedja A, Iacopetti I, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events?. Am J Transplant 2004; 4:475 - 481
- Byrne GW, Davies WR, Oi K, Rao VP, Teotia SS, Ricci D, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation 2006; 82:1787 - 1791
- Barth RN, Yamamoto S, LaMattina JC, Kumagai N, Kitamura H, Vagefi PA, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation 2003; 75:1615 - 1624
- Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, et al. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood 2004; 103:3964 - 3969
- Sykes M, Shimizu I, Kawahara T. Mixed hematopoietic chimerism for the simultaneous induction of T and B cell tolerance. Transplantation 2005; 79:28 - 29
- Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J Immunol 2004; 172:5143 - 5148
- Gianello P, Dufrane D. Encapsulation of pig islets by alginate matrix to correct streptozotocin-induced diabetes in primates without immunosuppression. Presented at the Joint meeting of the International Xenotransplantation Association (IXA), the International Pancreas and Islet Transplant Association (IPITA), and the Cell Transplant Society (CTS). Minneapolis, MN, USA. Xenotransplantation 2007; 14:441
- Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nature medicine 1995; 1:964 - 966
- Platt JL, Lin SS, McGregor CG. Acute vascular rejection. Xenotransplantation 1998; 5:169 - 175
- Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today 1996; 17:379 - 384
- Pino-Chavez G. Differentiating acute humoral from acute cellular rejection histopathologically. Graft 2001; 4:60 - 62
- Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation 1991; 52:214 - 220
- Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplantation proceedings 1992; 24:559 - 562
- Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1–3)Gal epitopes. Proc Natl Acad Sci USA 1993; 90:11391 - 11395
- McCurry KR, Kooyman DL, Alvarado CG, Cotterell AH, Martin MJ, Logan JS, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nature medicine 1995; 1:423 - 427
- Adams A, Pearson T, Larsen C. Conventional immunosuppression and co-stimulation blockade. Philos Trans R Soc Lond, B, Biol Sci 2001; 356:703 - 705
- Diamond LE, McCurry KR, Martin MJ, McClellan SB, Oldham ER, Platt JL, et al. Characterization of transgenic pigs expressing functionally active human CD59 on cardiac endothelium. Transplantation 1996; 61:1241 - 1249
- Schuurman HJ, Pino-Chavez G, Phillips MJ, Thomas L, White DJ, Cozzi E. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation 2002; 73:1146 - 1151
- Chen RH, Naficy S, Logan JS, Diamond LE, Adams DH. Hearts from transgenic pigs constructed with CD59/DAF genomic clones demonstrate improved survival in primates. Xenotransplantation 1999; 6:194 - 200
- Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. The American journal of pathology 2008; 172:1471 - 1481
- Lam TT, Hausen B, Hook L, Lau M, Higgins J, Christians U, et al. The effect of soluble complement receptor type 1 on acute humoral xenograft rejection in hDAF-transgenic pig-to-primate life-supporting kidney xenografts. Xenotransplantation 2005; 12:20 - 29
- Sharma A, Naziruddin B, Cui C, Martin MJ, Xu H, Wan H, et al. Pig cells that lack the gene for alpha1–3 galactosyltransferase express low levels of the gal antigen. Transplantation 2003; 75:430 - 436
- Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med 2005; 11:1295 - 1298
- Robson SC, Cooper DK, d'Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 2000; 7:166 - 176
- Buhler LH, Cooper DK. How strong is the T cell response in the pig-to-primate model?. Xenotransplantation 2005; 12:85 - 87
- Sebille F, Guillet M, Brouard S, Gagne K, Petzold T, Blancho G, et al. T-cell-mediated rejection of vascularized xenografts in the absence of induced anti-donor antibody response. Am J Transplant 2001; 1:21 - 28
- Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med 2005; 11:29 - 31
- Rayat GR, Rajotte RV, Hering BJ, Binette TM, Korbutt GS. In vitro and in vivo expression of Galalpha-(1,3)Gal on porcine islet cells is age dependent. J Endocrinol 2003; 177:127 - 135
- Bennet W, Sundberg B, Lundgren T, Tibell A, Groth CG, Richards A, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation 2000; 69:711 - 719
- Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low-molecular weight dextran sulfate abrogates the instant blood-mediated inflammatory reaction induced by adult porcine islets both in vitro and in vivo. Transplant Proc 2004; 36:1186 - 1187
- Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Kallen R, et al. Islet Surface Heparinization Prevents the Instant Blood-Mediated Inflammatory Reaction in Islet Transplantation. Diabetes 2007;
- Soderlund J, Wennberg L, Castanos-Velez E, Biberfeld P, Zhu S, Tibell A, et al. Fetal porcine islet-like cell clusters transplanted to cynomolgus monkeys: an immunohistochemical study. Transplantation 1999; 67:784 - 791
- Kirchhof N, Shibata S, Wijkstrom M, Kulick DM, Salerno CT, Clemmings SM, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation 2004; 11:396 - 407
- Solomon MF, Kuziel WA, Mann DA, Simeonovic CJ. The role of chemokines and their receptors in the rejection of pig islet tissue xenografts. Xenotransplantation 2003; 10:164 - 177
- Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006; 12:301 - 303
- Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 2006; 12:304 - 306
- Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation 2006; 81:1345 - 1353
- Cardona K, Milas Z, Strobert E, Cano J, Jiang W, Safley SA, et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant 2007; 7:2260 - 2268
- Koyama I, Kawai T, Andrews D, Boskovic S, Nadazdin O, Wee SL, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation 2004; 78:1238 - 1239
- Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest 1996; 98:1417 - 1422
- Dwyer KM, Mysore TB, Crikis S, Robson SC, Nandurkar H, Cowan PJ, D'Apice AJ. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation 2006; 82:428 - 432
- Klose R, Kemter E, Bedke T, Bittmann I, Kelsser B, Endres R, Pfeffer K, Schwinzer R, Wolf E. Expression of biologically active human TRAIL in transgenic pigs. Transplantation 2005; 80:222 - 230
- Tseng YL, Dor FJ, Kuwaki K, Ryan D, Wood J, Denaro M, et al. Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation 2004; 11:361 - 370
- Cosimi AB, Sachs DH. Mixed chimerism and transplantation tolerance. Transplantation 2004; 77:943 - 946
- Wood K. Is B cell tolerance essential for transplantation tolerance?. Transplantation 2005; 79:40 - 42
- Kawahara T, Shimizu I, Ohdan H, Zhao G, Sykes M. Differing mechanisms of early and late B cell hyporesponsiveness induced by mixed chimerism. Am J Transplant 2005; 5:2821 - 2829
- Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nature reviews 2007; 7:519 - 531
- Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 2005; 11:32 - 34
- Lee LA, Gritsch HA, Sergio JJ, Arn JS, Glaser RM, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proceedings of the National Academy of Sciences of the United States of America 1994; 91:10864 - 10867
- Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol 1999; 162:3402 - 3407
- Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Skin graft tolerance across a discordant xenogeneic barrier. Nature medicine 1996; 2:1211 - 1216
- Chen AM, Zhou Y, Swenson K, Sachs DH, Sykes M, Yang YG. Porcine stem cell engraftment and seeding of murine thymus with class II+ cells in mice expressing porcine cytokines: toward tolerance induction across discordant xenogeneic barriers. Transplantation 2000; 69:2484 - 2490
- Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 2006; 108:487 - 492
- Sykes M. Mixed chimerism and transplant tolerance. Immunity 2001; 14:417 - 424
- Mollnes TE, Fiane AE. Perspectives on complement in xenotransplantation. Mol Immunol 2003; 40:135 - 143
- Soin B, Smith KG, Zaidi A, Cozzi E, Bradley JR, Ostlie DJ, et al. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney international 2001; 60:1592 - 1597
- Schulte Am Esch J 2nd, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation 2005; 12:30 - 37
- Robson SC, Young VK, Cook NS, Metternich R, Kasper-Konig W, Lesnikoski BA, et al. Thrombin inhibition in an ex vivo model of porcine heart xenograft hyperacute rejection. Transplantation 1996; 61:862 - 868
- Siegel JB, Grey ST, Lesnikoski BA, Kopp CW, Soares M, Schulte am Esch J 2nd, Bach FH, Robson SC. Xenogeneic endothelial cells activate human prothrombin. Transplantation 1997; 64:888 - 896
- Kopp CW, Grey ST, Siegel JB, McShea A, Vetr H, Wrighton CJ, et al. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation 1998; 66:244 - 251
- Osborne FN, Kalsi KK, Lawson C, Lavitrano M, Yacoub MH, Rose ML, et al. Expression of human ecto-5′-nucleotidase in pig endothelium increases adenosine production and protects from NK cell-mediated lysis. Am J Transplant 2005; 5:1248 - 1255
- Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. The Journal of clinical investigation 2004; 113:1440 - 1446
- Chen D, Weber M, McVey JH, Kemball-Cook G, Tuddenham EG, Lechler RI, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant 2004; 4:1958 - 1963
- Toomey JR, Kratzer KE, Lasky NM, Broze GJ Jr. Effect of tissue factor deficiency on mouse and tumor development. Proceedings of the National Academy of Sciences of the United States of America 1997; 94:6922 - 6926
- Weiss EJ, Hamilton JR, Lease KE, Coughlin SR. Protection against thrombosis in mice lacking PAR3. Blood 2002; 100:3240 - 3244
- Mendicino M, Liu M, Ghanekar A, He W, Koscik C, Shalev I, Javadi M, et al. Targeted deletion of Fgl-2/fibroleukin in the donor modulates immunologic response and acute vascular rejection in cardiac xenografts. Circulation 2005; 112:248 - 256
- McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. The Journal of thoracic and cardiovascular surgery 2005; 130:844 - 851
- Palacios G, Druce J, Du L, Tran T, Birch C, Briese T, et al. A new arenavirus in a cluster of fatal transplant-associated diseases. The New England journal of medicine 2008; 358:991 - 998
- Fishman JA, Patience C. Xenotransplantation: infectious risk revisited. Am J Transplant 2004; 4:1383 - 1390
- Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science (New York, NY) 1999; 285:1236 - 1241
- Wilhelm M, Fishman JA, Pontikis R, Aubertin AM, Wilhelm FX. Susceptibility of recombinant porcine endogenous retrovirus reverse transcriptase to nucleoside and non-nucleoside inhibitors. Cell Mol Life Sci 2002; 59:2184 - 2190
- Oldmixon BA, Wood JC, Ericsson TA, Wilson CA, White-Scharf ME, Andersson G, et al. Porcine endogenous retrovirus transmission characteristics of an inbred herd of miniature swine. Journal of virology 2002; 76:3045 - 3048
- Scobie L, Taylor S, Wood JC, Suling KM, Quinn G, Meikle S, et al. Absence of replication-competent human-tropic porcine endogenous retroviruses in the germ line DNA of inbred miniature Swine. Journal of virology 2004; 78:2502 - 2509
- Fiebig U, Stephan O, Kurth R, Denner J. Neutralizing antibodies against conserved domains of p15E of porcine endogenous retroviruses: basis for a vaccine for xenotransplantation?. Virology 2003; 307:406 - 413
- Komoda H, Miyagawa S, Omori T, Takahagi Y, Murakami H, Shigehisa T, et al. Survival of adult islet grafts from transgenic pigs with N-acetylglucosaminyltransferase-III (GnT-III) in cynomolgus monkeys. Xenotransplantation 2005; 12:209 - 216
- Dieckhoff B, Petersen B, Kues WA, Kurth R, Niemann H, Denner J. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation 2008; 15:36 - 45
- Wang D, Coscoy L, Zylberberg M, Avila PC, Boushey HA, Ganem D, et al. Microarray-based detection and genotyping of viral pathogens. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:15687 - 15692
- U.S. Public Health Service. PHS Guideline on Infectious Disease Issues in Xenotransplantation 2001; www4.od.nih.gov/oba/sacx/xenoguide01.pdf
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research (CBER). Guidance for Industry—Source Animal, Product, Preclinical and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans 2003; April http://www.fda.gov/cber/guidelines.htm
- Council of Europe Committee of Ministers. Recommendation Rec(2003)10 of the Committee of Ministers to Member States on Xenotransplantation 2003; http://www.legaltext.ee/text/en/K90038.htm
- World Health Organisation. Statement from the xenotransplantation advisory consultation 2005; April Geneva 18–20 http://www.who.int/transplantation/xeno/en/
- Sykes M, d'Apice A, Sandrin M. Position paper of the Ethics Committee of the International Xenotransplantation Association. Xenotransplantation 2003; 10:194 - 203
- Sykes M, Sandrin M, D'Apice A. Guidelines for xenotransplantation. The New England journal of medicine 2003; 349:1294 - 1295
- Sykes M, Sandrin M, Cozzi E, Rees MA. World Health Organization resolution on xenotransplantation. Xenotransplantation 2004; 11:224 - 225
- d'Apice AJ, Cowan PJ. Gene-modified pigs. Xenotransplantation 2008; 15:87 - 90
- Nottle MB, Beebe LF, Harrison SJ, McIlfatrick SM, Ashman RJ, O'Connell PJ, et al. Production of homozygous alpha-1,3-galactosyltransferase knockout pigs by breeding and somatic cell nuclear transfer. Xenotransplantation 2007; 14:339 - 344
- Lavitrano M, Busnelli M, Cerrito MG, Giovannoni R, Manzini S, Vargiolu A. Sperm-mediated gene transfer. Reproduction, fertility and development 2006; 18:19 - 23
- Martin C, Plat M, Nerriere-Daguin V, Coulon F, Uzbekova S, Venturi E, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic research 2005; 14:373 - 384