Abstract
A case of L-type-like atypical bovine spongiform encephalopathy was detected in 14-year-old Japanese black beef cattle (BSE/JP24). To clarify the biological and biochemical properties of the prion in BSE/JP24, we performed a transmission study with wild-type mice and bovinized transgenic mice (TgBoPrP). The BSE/JP24 prion was transmitted to TgBoPrP mice with the incubation period of 199.7 ± 3.4 days, which was shorter than that of classical BSE (C-BSE) (223.5 ± 13.5 days). Further, C-BSE was transmitted to wild-type mice with the incubation period of about 409 days, whereas BSE/JP24 prion inoculated mice showed no clinical signs up to 649 days. Severe vacuolation and a widespread and uniform distribution of PrPSc were pathologically observed in the brain of BSE/JP24 prion affected TgBoPrP mice. The molecular weight and glycoform ratio of PrPSc in BSE/JP24 were different from those in C-BSE, and PrPSc in BSE/JP24 exhibited weaker proteinase K resistance than that in C-BSE. These findings revealed that the BSE/JP24 prion has distinct biological and biochemical properties reported for that of C-BSE. Interestingly, a shorter incubation period was observed at the subsequent passage of the BSE/JP24 prion to TgBoPrP mice (152.2 ± 3.1 days). This result implies that BSE/JP24 prion has newly emerged and showed the possibility that L-type BSE prion might be classified into multiple strains.
Introduction
Bovine spongiform encephalopathy (BSE) is a type of transmissible spongiform encephalopathy (TSE) or prion disease,Citation1 and it causes variant Creutzfeld-Jakob disease (vCJD) in humans.Citation2,Citation3 TSEs are characterized by the accumulation of an abnormal prion protein (PrPSc), which is a protease-resistant isoform of the host-encoded cellular prion protein (PrPC), in the affected brain. According to the protein-only hypothesis, PrPSc is the principal component of the infectious agent. Prion strain classification is based on the result of mice transmission, e.g., incubation period, lesion profile and PrPSc deposition pattern.Citation4–Citation7 Recently, the biochemical characteristics of PrPSc have been also used to discriminate prion strains.Citation8–Citation12
In recent years, several cases of atypical neuropathological and molecular phenotypes of BSE (atypical BSE) have been reported in Japan, several European countries and North America.Citation13–Citation19 Atypical BSE cases are temporally classified into two groups—L-type and H-type—according to the molecular size of the proteinase K (PK) digested non-glycosylated form of PrPSc.Citation18 However, the precise definition of atypical BSE prions remains to be established.
In Japan, two atypical BSE cases were observed. One was a case of L-type BSE found in healthy 23-month-old steer Holstein (BSE/JP8).Citation13 In this case, PrPSc was present in a very small amount in the brain, thereby preventing successful transmission to bovinized transgenic mice (TgBoPrP).Citation20 The other was found in 14-year-old Japanese black beef cattle (BSE/JP24).Citation21 PrPSc in this case showed a similar glycoform ratio to that of Italian L-type BSE [bovine amyloidotic spongiform encephalopathy (BASE)] or human sporadic Creutzfeldt-Jacob disease (type-2) in the western blot analysis. However, unlike BASE, the non-glycosylated form of PrPSc in BSE/JP24 did not exhibit a clear faster migration as compared with that of classical BSE cases.Citation21 In this case, a considerable amount of PrPSc accumulated in the brain. However, the transmissibility and the precise characteristics of the BSE/JP24 prion remain elusive. To clarify the biological and biochemical properties of the BSE/JP24 (L-type-like) prion, we performed a transmission study with wild-type mice and TgBoPrP mice. This study showed the intensive transmissibility of BSE/JP24 prion to TgBoPrP mice. We found that BSE/JP24 prion has biological and biochemical properties distinct from those of C-BSE prion, and BSE/JP24 prion has not completely adapted to bovinized mice.
Results
Transmissibility of BSE/JP24 prion to mice.
TgBoPrP mice inoculated with brain homogenates of BSE/JP24 and C-BSE developed a progressive neurological disease with incubation periods of 199.7 ± 3.4 and 223.5 ± 13.5 days, respectively (). The subsequent passage of the brain homogenate of BSE/JP24 prion affected TgBoPrP showed a shorter incubation period of 152.2 ± 3.1 days. In contrast, the incubation period of the second passage of C-BSE prion in TgBoPrP was 214.9 ± 4.8 days, which was similar to the period observed in the first passage (). These two cattle samples were also inoculated intracerebrally into ICR (wild-type) mice. While C-BSE prion was transmitted to ICR mice with an incubation period of 408.6 days, BSE/JP24 prion inoculated ICR mice showed no abnormality up to 649 days post inoculation. No PrPSc accumulation and vacuolation was observed at 649 days post inoculation (data not shown).
Molecular mass and glycoform of PrPSc in BSE/JP24 prion passaged TgBoPrP mice.
All the diseased TgBoPrP mice inoculated with BSE/JP24 prion harboured PrPSc in their brains. Before transmission to the TgBoPrP mice, the non-glycosylated form of PK digested PrPSc of BSE/JP24 showed indistinguishable mobility with that of C-BSE (, lanes 1 and 2) in western blot analysis as reported previously.Citation21 However, when PrPSc was treated with PNGaseF, we attained improved resolution and observed a slightly faster migration pattern of the non-glycosylated form of PrPSc of BSE/JP24 than that of C-BSE (, lanes 3 and 4). Intriguingly, this difference became more apparent after transmission to TgBoPrP mice (, lanes 5 to 8). PrPSc from BSE/JP24 showed a glycoform pattern distinct from that of C-BSE ( and B). This different glycoform pattern was conserved in BSE/JP24 prion transmitted TgBoPrP mice ().
Neuropathological examination.
The BSE/JP24 prion affected TgBoPrP mice showed a higher score and a different lesion profile when compared to those of C-BSE prion affected TgBoPrP mice (). Dense PrPSc deposition was observed in particular nuclei in the pons (pontine and vestibular nuclei), midbrain (interstitial nucleus and red nucleus), thalamus (habenular nuclei) and cerebellum or the adjacent periventricular area in the frontal lobe and the striatum of the C-BSE prion affected TgBoPrP mice (). Fine and coarse granular PrPSc was predominant in the C-BSE prion affected TgBoPrP mice (). PrP plaques were observed in the adjacent periventricular area in the frontal lobe, oriens layer of hippocampus and corpus callosum of C-BSE prion affected TgBoPrP mice (inset of and C). In contrast, fine punctuate and fine granular PrPSc was dominant in BSE/JP24 prion affected TgBoPrP mice (). The topographical distribution of PrPSc deposits was dense and uniform in the pons, cerebellar medulla, midbrain, thalamus and corpus callosum ). No PrP plaque was present in the BSE/JP24 prion affected TgBoPrP mice ( and D). Similar PrPSc deposits and distribution patterns were observed in the first and second passages of BSE/JP24 prion affected TgBoPrP mice (data not shown). These results revealed striking differences between C-BSE and BSE/JP24 prion affected TgBoPrP mice.
Relative PK resistance of PrPSc from C-BSE and BSE/JP24.
The relative PK resistance of PrPSc from BSE/JP24 and C-BSE was analyzed. The PrPSc concentration of the sample was adjusted by prion affected TgBoPrP mice weakened slightly at the second passage (), no significant difference was observed statistically (data not shown).
Conformational stability studies.
The Western blot signal intensity showed that [GdnHCl]1/2 for denaturation of PrPSc in cattle was 3.1 ± 0.1 M (C-BSE) and 2.9 ± 0.3 M (BSE/JP24). The conformation stability of the C-BSE and the BSE/JP24 prion affected TgBoPrP mice were 2.9 ± 0.2 M and 2.8 ± 0.1 M at the first passage and 3.0 ± 0.2 M and 2.8 ± 0.2 M at the second passage, respectively. No clear difference was observed between the [GdnHCl]1/2 values in BSE/JP24 and C-BSE ().
Discussion
Our study showed the biological and biochemical features of BSE/JP24 prion. The incubation period of BSE/JP24 prion inoculated TgBoPrP mice was shorter than that of C-BSE prion (). All TgBoPrP mice that succumbed to BSE/JP24 prion possessed PrPSc in their brains. A different glycoform of PrPSc with relatively weaker PK resistance, was detected in passaged mice, similar as the observation in the case of cattle ( and ). Histopathologically, BSE/JP24 prion affected TgBoPrP mice exhibited severe vacuolation and widespread and uniform PrPSc deposition in the brain (). Furthermore, C-BSE prion was transmitted to wild-type mice, while BSE/JP24 prion was not up to 649 days (). These finding showed that BSE/JP24 and C-BSE prion have distinct biological and biochemical properties. We conclude that the BSE/JP24 prion is different from the C-BSE one.
Recently, L-type BSE cases were detected in several countries,Citation13,Citation14,Citation16,Citation18,Citation19 and their characteristics were investigated.Citation16,Citation20,Citation30,Citation31 It has been reported that the European L-type BSE has a distinct glycoform of PrPSc that has relatively weaker PK resistance.Citation18 These characteristics closely resemble with those of BSE/JP24 ().Citation21 In addition, similar incubation periods, spongiform changes and PrPSc deposition patterns were observed in BSE/JP24 and European L-type BSE prion affected bovinized transgenic mice ( and ).Citation16,Citation30,Citation31 We also showed that the lesion profiles and incubation periods in the C-BSE prion inoculated TgBoPrP were similar to those of C-BSE prion inoculated Tgbov XV ( and ).Citation30 Thus, the biological characteristics of BSE/JP24 prion seemed to be similar to those of European L-type BSE prion. However, BSE/JP24 prion affected cattle exhibited considerable PrPSc deposition at the obex, similar to the observation with C-BSE,Citation21 while in the case of the Italian L-type BSE (BASE), a European L-type BSE, weak PrPSc positivity was observed in the brainstem.Citation14 The lesion profile of BSE/JP24 prion affected TgBoPrP was slightly different from that of BASE prion inoculated Tgbov XV, although both showed similar incubation periods and PrPSc deposition patterns ().Citation30 These findings may suggest that BSE/JP24 prion has different characters from L-type BSE in Europe.
For further characterization, we performed a second passage of BSE/JP24 prion to TgBoPrP mice. Although no significant differences were observed in the lesion profile and PrPSc deposition between the first and the second passaged mice (), the incubation periods became shorter (). This phenomenon had not been reported in European L-type BSE.Citation31 The amount of PK-digested PrPSc in BSE/JP24 cattle and C-BSE cattle, together with the amount of passaged in TgBoPrP, were not different (). Thus, we can rule out the possibility that the lower prion titer of BSE/JP24 cattle caused the longer incubation period in the first passage. This result suggests that the primary passaged BSE/JP24 prion was not fully adapted to TgBoPrP mice. This implies that the BSE/JP24 prion may have emerged newly and it may not have completely adapted to cattle species. The different glycoform of PrPSc was observed in the C-BSE prion second passaged TgBoPrP with a high concentration of PK digestion (). This result may suggest that PrPSc with different characteristics had emerged depending on passage. However, the biological properties, as represented by the incubation period, did not change in C-BSE prion.
In this study, we examined the detailed characteristics of the prions in the Japanese L-type-like BSE case, and showed the possibility that L-type BSE prion might be classified into multiple strains. Further characterization with transmission study including the comparative analysis of L-type BSE prions from the different countries may necessary to be cleared their origins.
Methods
BSE materials.
The brain sample of L-type-like BSE (BSE/JP24) affected cattle was used in this study.Citation21 Classical BSE affected brain sample was also used which was kindly provided by the Veterinary Laboratories Agency (VLA), Weybridge, UK. These brain samples were stored at −80°C until use.
Transmission studies.
The transmissibility of BSE/JP24 and classical BSE prion were assessed by using transgenic mice expressing bovine PrP [Tg(BoPrP) 4092HOZ/Prnp0/0; TgBoPrP] and wild-type mice (ICR: Japan SLC, Inc.,). TgBoPrP mice, which are highly susceptible to C-BSE prions, were supplied by Dr. S.B. Prusiner.Citation22 The brain of C-BSE and BSE/JP24 prion affected cattle were homogenized in nine volumes of phosphate buffered saline (PBS) using a multi-bead shocker (Yasui Kikai) and centrifuged at 1,000 xg for 5 min at room temperature (RT). The supernatant was used as the inoculum. Female TgBoPrP mice (3-week-old) were inoculated intracerebrally with 20 µl of supernatant. After inoculation, the clinical status of the mice was monitored daily to assess the onset of neurological signs. Diseased mice were sacrificed and subjected to PrPSc examination as described previously.Citation23
PrPSc extraction from the brain(s) of BSE prion affected cattle and mice.
PrPSc was extracted from the cattle brain tissues by a method described previously.Citation24 Briefly, the brain tissues of cattle were homogenized at 20% concentration (wt/vol) in a buffer containing 100 mM NaCl and 50 mM Tris-HCl (pH 7.6). The brain homogenate (250 µl) were mixed with an equal volume of detergent buffer containing 4% (wt/vol) Zwittergent 3–14 (Calbiochem), 1% (wt/vol) Sarkosyl, 100 mM NaCl and 50 mM Tris-HCl (pH 7.6), and incubated with 6.25 µl of 40 mg/ml collagenase. Then, the sample was subjected to proteinase K (PK; Roche Diagnostic) digestion at various concentrations (40–1,000 µg/ml) to evaluate the PK resistance of PrPSc. PK digestion was terminated with 2 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (Pefabloc; Roche Diagnostics). The sample was mixed with a 2-butanol: methanol mixture (5:1) and centrifuged at 20,000 xg for 10 min. PrPSc was detected in the brain of mice according to a method described previously.Citation23 For the detection of PrPSc in mice brains, 10% of brain homogenate was mixed with an equal volume of detergent buffer containing 0.01% Triton X-100, 0.01% sodium deoxycholate, 100 mM NaCl and 10 mM Tris-HCl (pH 7.6), and then incubated with PK at various concentrations (40–1,000 µg/ml). After PK treatment, some samples were deglycosylated with N-glycosidase F (PNGaseF; New England Biolabs) following the manufacturer's instructions.
Western blotting analysis.
The extracted samples were mixed with a gel-loading buffer containing 2% (wt/vol) SDS and were boiled for 5 min before electrophoresis. The sample was separated by 12% SDS-PAGE and blotted electrically onto a PVDF membrane (Millipore). The blotted membrane was incubated with anti-PrP monoclonal antibody (mAb) 6H4 (Prionics) at RT for 1 h. Signals were developed with a chemiluminescent substrate (SuperSignal; Pierce biotechnology). The glycoform ratio of PrPSc was calculated by using the Fluorochem software (Alpha-Innotech Co.,).
Conformational stability assay.
Conformational stability assay was performed according to methods described previously.Citation25 Briefly, 50 µl of 10% brain homogenate was added to an equal volume of guanidine hydrochloride (GdnHCl) with a concentration range of 0 to 8 M. Mixed samples were incubated at 37°C for 1 h. Then, the sample was diluted by the addition of 850 µl of Tris buffer containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% Triton-X 100 and 0.5% deoxycholate. Then 50 µl of GdnHCl was added to each sample to obtain a final concentration to 0.4 M. Next, the samples were digested with 20 µg/ml PK at 37°C for 1 h. PrPSc concentration and Western blotting analysis were carried out as described above. Conformation stability was examined by using mAb 6H4 recognized PrP amino acid residues 143–151. The GdnHCl concentration at half maximal denaturation ([GdnHCl]1/2) was used as a measure of the relative conformational stability of PrPSc. [GdnHCl]1/2 was calculated based on the denaturation curves obtain by densitometric analysis using the Fluorochem software (Alpha Innotech Co.,).
Neuropathological examination.
The brain was rapidly removed from mice that were killed at the terminal stage of the disease. The brain was then fixed in 10% buffered formalin solution (pH 7.4). Coronal slices of the brain were immersed in 98% formic acid, to reduce the infectivity for 1 h at RT,Citation26 and embedded in paraffin wax. Sections with a thickness of 4 µm were cut and stained with haematoxylin and eosin (HE) or immunohistochemistry. For neuropathological analysis, the lesion profile was determined using the HE-stained sections by scoring the vacuolar changes in nine standard grey matter areas, as previously described.Citation27
Immunohistochemistry.
The sections were incubated in 3% hydrogen peroxide for 15 min, treated with 10 µg/ml of PK (Wako Pure Chemical) for 10 min, and incubated in 100 mM sodium hydroxide at 60°C for 10 min. The sections were then incubated in anti-PrP monoclonal antibody mAb F99/97.6.1 (VMRD, Inc.,). Immunostaining was performed using a Nichirei Histo-fine MAX-PO (M) kit (Nichirei), with 3-3′-diaminobenzidine tetrachloride as the chromogen. Finally, the sections were counterstained with haematoxylin.
PET blot.
Paraffin embedded tissue (PET) blot was performed as described previouslyCitation28,Citation29 with the following modification. Dewaxed membranes were treated with 80 µg/ml of PK for 30 min at 37°C followed by denaturation using 3 M guanidine thiocyanate for 10 min at RT. The membranes were then incubated with primary mAb SAF84 (SPI-bio) for 90 min at RT. Then, they were incubated in alkaline phosphatase-coupled anti-mouse antibody [1:250, Nichirei Histofine Simple Stain MAX-AP (M) (Nichirei)] and visualized with 5-bromo 4-chloro 3-indolyl phosphate and nitroblue tetrazolium (BCIP/NBT; Roche Diagnostics) as a substrate.
Abbreviations
| BSE | = | bovine spongiform encephalopathy |
| TSE | = | transmissible spongiform encephalopathy |
| C-BSE | = | classical BSE |
| L-type BSE | = | low-type BSE |
| H-type BSE | = | high-type BSE |
| BASE | = | bovine amyloidotic spongiform encephalopathy |
| CJD | = | Creutzfeldt-Jakob disease |
| BSE/JP24 | = | the 24th BSE case in Japan |
| PrPSc | = | abnormal isoform of prion protein |
| PrPC | = | cellular isoform of prion protein |
| TgBoPrP | = | bovinized transgenic mice |
| PET | = | paraffin embedded tissue |
| mAb | = | monoclonal antibody |
| PK | = | proteinase K |
| PNGaseF | = | N-glycosidase F |
| SDS | = | sodium dodecyl sulfate |
Figures and Tables
Figure 1 Western blotting analysis of PrPSc from C-BSE and BSE/JP24. (A) The fragment of PrPSc in the cattle brain of C-BSE (lanes 1 and 3) and BSE/JP24 (lanes 2 and 4). PrPSc in the brain of TgBoPrP inoculated with C-BSE prion (lanes 5 and 7) and BSE/JP24 prion (lanes 6 and 8). All samples were digested with 50 µg/ml PK at 37°C for 1 h, and then samples in lanes 3, 4, 7 and 8 were treated with PNGaseF. PrPSc was detected by using mAb 6H4. Molecular markers are shown on the left (kDa). (B) The relative amount of the di-, mono- and non-glycosylated form of PrPSc in the C-BSE and BSE/JP24 prion affected individual. The results are mean ± standard deviation in five experiments. Bar diagram: diglycosylated form (black), monoglycosylated form (grey) and nonglycosylated form (white).
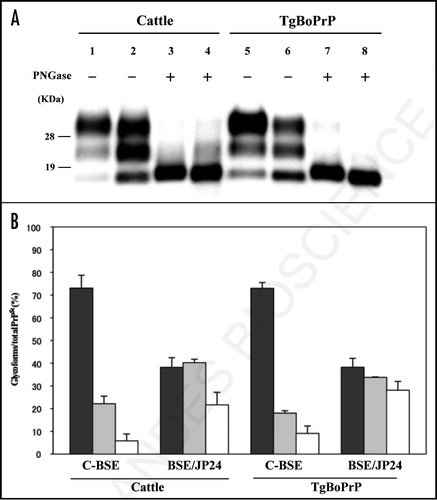
Figure 2 Lesion profile of TgBoPrP mice inoculated with C-BSE and BSE/JP24 prions. Vacuolation was scored on a 0–5 (mean values) in the following brain areas: 1, dorsal medulla; 2, cerebellar cortex; 3, superior cortex; 4, hypothalamus; 5, thalamus; 6, hippocampus; 7, septal nuclei of the paraterminal body; 8, cerebral cortex at the levels of 4 and 5; and 9, cerebral cortex at the level of 7. •: 1st passage of BSE/JP24 prion, ○: 2nd passage of BSE/JP24 prion, ▴: 1st passage of C-BSE prion.
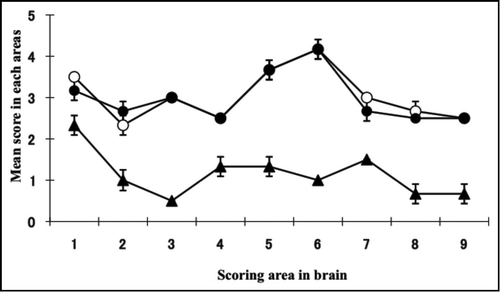
Figure 3 Histopathological (A and B), immunohistochemical (C and D) and PET-blot (E and F) analysis of TgBoPrP mice inoculated with C-BSE and BSE/JP24 prions. No distinct vacuolation in the presence of PrP plaques was detected in the cerebral cortex and hippocampal region in C-BSE prion affected TgBoPrP mice (A), whereas severe vacuolation in the absence of PrP-positive deposits was prominent in BSE/JP24 prion affected TgBoPrP mice (B). Immunolabelled PrPSc showed coarse granular and coalescing-like patterns in the gigantocellular nucleus of medulla oblongata of C-BSE prion affected TgBoPrP mice (C). A diffused fine granular pattern was observed in BSE/JP24 prion affected TgBoPrP mice (D). Immunohistochemical labelling with mAb F99/97.6.1. The insets at the lower right corner (A and C) are PrP plaques detected in the periventricular area of frontal lobe (arrowheads). PET blot reveals that immunolabelled PrPSc was marked in particular nuclei of brainstems in C-BSE prion affected TgBoPrP mice (E). On the other hand, widespread and homogeneous PrPSc immunolabelling was obvious in BSE/JP24 prion affected TgBoPrP mice (F). PET blots of the representative coronal section at the level of the hippocampus (left) and medulla oblongate (right) are shown. The mAb SAF84 was used in PET-blot analysis. Bar: 200 µm (A and B); 50 µm (C and D); 20 µm (inset of A and C).
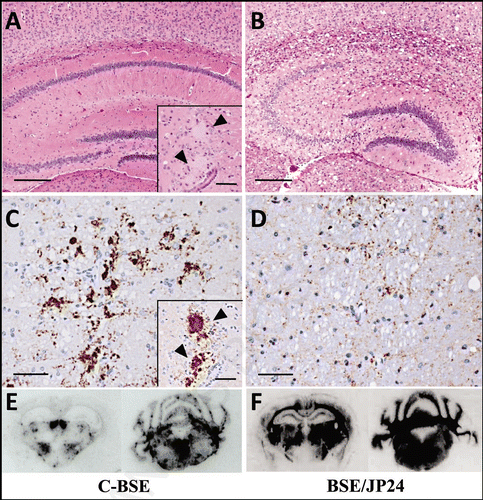
Figure 4 Relative PK resistance of PrPSc in prion affected cattle and TgBoPrP mice. (A) PK resistance of PrPSc in the cattle brain of C-BSE and BSE/JP24. (B) PK resistance of PrPSc of TgBoPrP mice inoculated with C-BSE and BSE/JP24 prions. (C) PK resistance of PrPSc from the subsequent passage of C-BSE and BSE/JP24 prion to TgBoPrP mice. The PrPSc concentration of sample was adjusted by the signal intensity of western blotting. The samples were treated with PK of various concentration (40–1,000 µg/ml) at 37°C for 1 h. Data shown represent one of three experiments demonstrating similar trends. PrPSc was detected with mAb 6H4. Molecular markers are shown on the left (kDa).
Table 1 Transmission of C-BSE and BSE/JP24 prion to mice
Table 2 Conformational stability ([GdnHCl]1/2 value) of PrPSc from C-BSE and BSE/JP24
Acknowledgements
We thank Ms. Naoko Tabeta, Shuko Kodani, Nozomi Matsumoto, Atsuko Ojima, Ritsuko Miwa and Mutsumi Sakurai for their technical assistance. Further, we thank Dr. Morikazu Shinagawa for his encouragement; Ms. Junko Yamada for her general assistance; and Mr. Manabu Aida, Ms. Chizuru Kuramochi, Che Jing Zh and the animal laboratory staff at the National Institute of Animal Health for maintaining the mouse colony. This study was supported in part by a Grant-in-Aid from the Bovine Spongiform Encephalopathy Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan; a grant for BSE research from the Ministry of Health, Labour and Welfare of Japan; and a grant-in-aid for the strategic cooperation to control emerging and reemerging infection from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Prusiner SB. Molecular biology of prion diseases. Science 1991; 252:1515 - 1522
- Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, et al. The same prion strain causes vCJD and BSE. Nature 1997; 389:448 - 450
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 1997; 389:498 - 501
- Bruce ME, Dickinson AG. Biological evidence that scrapie agent has an independent genome. J Gen Virol 1987; 68:79 - 89
- Kimberlin RH, Walker CA, Fraser H. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol 1989; 70:2017 - 2025
- Bruce ME. Scrapie strain variation and mutation. Br Med Bull 1993; 49:822 - 838
- Hirogari Y, Kubo M, Kimura KM, Haritani M, Yokoyama T. Two different scrapie prions isolated in Japanese sheep flocks. Microbiol Immunol 2003; 47:871 - 876
- Bessen RA, Marsh RF. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol 1992; 66:2096 - 2101
- Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant' CJD. Nature 1996; 383:685 - 690
- Kuczius T, Groschup MH. Differences in proteinase K resistance and neuronal deposition of abnormal prion proteins characterize bovine spongiform encephalopathy (BSE) and scrapie strains. Mol Med 1999; 5:406 - 418
- Parchi P, Capellari S, Chen SG, Petersen RB, Gambetti P, Kopp N, et al. Typing prion isoforms. Nature 1997; 386:232 - 234
- Somerville RA, Chong A, Mulqueen OU, Birkett CR, Wood SC, Hope J. Biochemical typing of scrapie strains. Nature 1997; 386:564
- Yamakawa Y, Hagiwara K, Nohtomi K, Nakamura Y, Nishijima M, Higuchi Y, et al. Atypical proteinase K-resistant prion protein (PrPres) observed in an apparently healthy 23-month-old Holstein steer. Jpn J Infect Dis 2003; 56:221 - 222
- Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, et al. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci USA 2004; 101:3065 - 3070
- Biacabe AG, Laplanche JL, Ryder S, Baron T. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep 2004; 5:110 - 114
- Buschmann A, Gretzschel A, Biacabe AG, Schiebel K, Corona C, Hoffmann C, et al. Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet Microbiol 2006; 117:103 - 116
- Richt JA, Kunkle RA, Alt D, Nicholson EM, Hamir AN, Czub S, Kluge J, et al. Identification and characterization of two bovine spongiform encephalopathy cases diagnosed in the United States. J Vet Diagn Invest 2007; 19:142 - 154
- Jacobs JG, Langeveld JP, Biacabe AG, Acutis PL, Polak MP, Gavier-Widen D, et al. Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. J Clin Microbiol 2007; 45:1821 - 1829
- Polak MP, Zmudzinski JF, Jacobs JG, Langeveld JP. Atypical status of bovine spongiform encephalopathy in Poland: a molecular typing study. Arch Virol 2008; 153:69 - 79
- Yokoyama T, Masujin K, Yamakawa Y, Sata T, Murayama Y, Shu Y, et al. Experimental transmission of two young and one suspended bovine spongiform encephalopathy (BSE) cases to bovinized transgenic mice. Jpn J Infect Dis 2007; 60:317 - 320
- Hagiwara K, Yamakawa Y, Sato Y, Nakamura Y, Tobiume M, Shinagawa M, et al. Accumulation of mono-glycosylated form-rich, plaque-forming PrPSc in the second atypical bovine spongiform encephalopathy case in Japan. Jpn J Infect Dis 2007; 60:305 - 308
- Scott MR, Safar J, Telling G, Nguyen O, Groth D, Torchia M, et al. Identification of a prion protein epitope modulating transmission of bovine spongiform encephalopathy prions to transgenic mice. Proc Natl Acad Sci USA 1997; 94:14279 - 14284
- Yokoyama T, Kimura KM, Ushiki Y, Yamada S, Morooka A, Nakashiba T, et al. In vivo conversion of cellular prion protein to pathogenic isoforms, as monitored by conformation-specific antibodies. J Biol Chem 2001; 276:11265 - 11271
- Hayashi HK, Yokoyama T, Takata M, Iwamaru Y, Imamura M, Ushiki YK, et al. The N-terminal cleavage site of PrPSc from BSE differs from that of PrPSc from scrapie. Biochem Biophys Res Commun 2005; 328:1024 - 1027
- Legname G, Nguyen HO, Baskakov IV, Cohen FE, Dearmond SJ, Prusiner SB. Strain-specified characteristics of mouse synthetic prions. Proc Natl Acad Sci USA 2005; 102:2168 - 2173
- Taylor DM, Brown JM, Fernie K, McConnell I. The effect of formic acid on BSE and scrapie infectivity in fixed and unfixed brain-tissue. Vet Microbiol 1997; 58:167 - 174
- Fraser H, Dickinson AG. The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol 1968; 78:301 - 311
- Schulz-Schaeffer WJ, Tschoke S, Kranefuss N, Drose W, Hause-Reitner D, et al. The paraffin-embedded tissue blot detects PrPSc early in the incubation time in prion diseases. Am J Pathol 2000; 156:51 - 56
- Lezmi S, Bencsik A, Baron T. PET-blot analysis contributes to BSE strain recognition in C57Bl/6 mice. J Histochem Cytochem 2006; 54:1087 - 1094
- Capobianco R, Casalone C, Suardi S, Mangieri M, Miccolo C, Limido L, et al. Conversion of the BASE prion strain into the BSE strain: the origin of BSE?. PLoS Pathog 2007; 3:31
- Beringue V, Andreoletti O, Le Dur A, Essalmani R, Vilotte JL, Lacroux C, et al. A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J Neurosci 2007; 27:6965 - 6971