Abstract
Prion diseases are fatal, neurodegenerative diseases characterized by the structural conversion of the normal, cellular prion protein, PrPC into an abnormally structured, aggregated and partially protease-resistant isoform, termed PrPSc. Although substantial research has been directed toward development of therapeutics targeting prions, there is still no curative treatment for the disease. Benzoxazines are bicyclic heterocyclic compounds possessing several pharmaceutically important properties, including neuroprotection and reactive oxygen species scavenging. In an effort to identify novel inhibitors of prion formation, several 5,7,8-trimethyl-1,4-benzoxazine derivatives were evaluated in vitro for their effectiveness on the expression levels of normal PrPC and its conversion to the abnormal isoforms of PrPSc in a scrapie-infected cell culture model. The most potent compound was 2-(4-methoxyphenyl)-5,7,8-trimethyl-3,4-dihydro-2H-1,4-benzoxazine, with a diminishing effect on the formation of PrPSc, thus establishing a class of compounds with a promising therapeutic use against prion diseases.
Introduction
Transmissible Spongiform Encephalopathies are fatal neurodegenerative diseases that include Creutzfeldt-Jakob Disease (sCJD, vCJD) in humans, scrapie in sheep and goats and Bovine Spongiform Encephalopathy (BSE) in cattle. Prion diseases typically exhibit a lengthy latency period between the time of infection and clinical manifestation.Citation1 Despite substantial research on prion diseases, key issues, including the prion pathogenic mechanism and TSE transmissibility, remain elusive. Currently, there are no effective therapies for TSEs. Therefore, it is of crucial importance to identify compounds with therapeutic or prophylactic activity against these diseases.
The key event in prion pathogenesis is the structural conversion of the normal, glycolipid-anchored host prion protein, PrPC into a misfolded conformational isoform, termed PrPSc.Citation2 Although PrPC is easily digested with proteinase K, the pathological form, PrPSc, displays partial PK-resistance. Inhibition of PrPSc formation has been a primary target for therapeutic intervention against TSEs.Citation3,Citation4 Cell lines chronically infected with TSEs have been efficiently used to study prion-related cellular processes,Citation5 as well as to screen for potentially effective anti-prion compounds. Among others, chronically scrapie-infected neuroblastoma cells (ScN2a) have been used extensively as a relevant model for studying TSEs, since they are able to promote stable and persistent replication of PrPSc,Citation6 upon subpassaging.
Several compounds have been tested for anti-prion activity in vitro and in vivo.Citation7 The most effective strategies for discovering such compounds include screening for inhibitors of PrPSc accumulation in infected cell lines, eliminating physiological PrPC as the substrate for prion conversion, and enhancing PrPSc degradation.Citation8 A variety of larger molecules such as pentosan polysulfate,Citation9 suramin,Citation10 amphotericin B,Citation11,Citation12 Congo redCitation13 and dendritic polyaminesCitation14 have been shown to possess antiprion properties. Among small molecules, bis-acridines,Citation15 polyphenols, phenothiazines, antihistamines, statins, some antimalarial agents,Citation16,Citation17 indole-3-glyoxamides,Citation18 pyridyl hydrazonesCitation19 and 2-aminothiazolesCitation20 have been shown to inhibit PrPSc formation in vitro. However, none of the aforementioned compounds has been proven to be effective in animal models when administered during the onset of neurological symptoms.
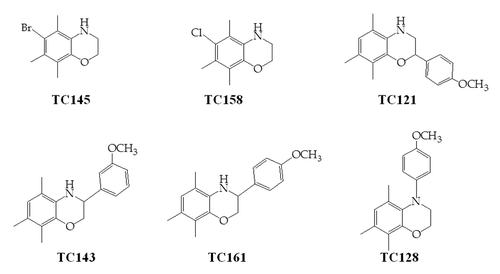
The 2H-1,4-benzoxazine-3-(4H)-one and 3,4-dihydro-2H-1,4-benzoxazine derivatives have been studied extensively for their ability to act as biologically active compounds including their role as neuroprotectants.Citation21 Our research activities have focused on the design and synthesis of bioactive compounds possessing the 5,7,8-trimethyl-1,4-benzoxazine core which can be considered as a classical bioisostere of the 5,7,8-trimethylbenzopyran nucleus of the well-known chain-breaking antioxidant vitamin E.Citation22 In the course of our efforts to explore the biological properties of this class of compounds we examined six derivatives () for their effectiveness on inhibition of PrPSc formation in mouse neuroblastoma cells (N2a)Citation23 permanently infected with the mouse adapted scrapie strain 22L. 2-(4-methoxyphenyl)-5,7,8-trimethyl-3,4-dihydro-2H-1,4-benzoxazine (TC121) possesses an EC50 of 1.17 μΜ and an EC90 of 3.2 μΜ. Our data clearly indicate TC121 as a lead structure, capable of reducing PrPSc concentrations in ScN2a cells, a finding of potential therapeutic relevance.
Results
Screening for PrPSc inhibitors
In this study we used 22L-scrapie-infected mouse neuroblastoma cells, a well-established in vitro prion disease model, to screen 5,7,8-trimethyl-1,4-benzoxazine derivatives for a potential effect on PrPC and PrPSc levels. The structures of the compounds of the present study are shown in . Initially, we verified the endogenous ability of the cells to continuously convert PrPC into PrPSc as assessed by western blot following PK digestion and we compared this PrP immunostaining pattern to that obtained following PK treatment of TSE-infected rodent brain homogenates (data not shown). Before assessing the influence of the derivatives on prion replication, we examined their effect on viability of the 22L scrapie N2a cells by cytometry and calculated the pertinent LD50 (50% lethal dose) values (). In order to evaluate the potential effect of our derivatives on PrPSc formation, we incubated 22L-ScN2a cells with the compounds as indicated in Materials and Methods. Cell extracts were treated with PK to enrich them in PrPSc and PrP-immunolabeling was compared between control and drug treated cells. Assessment of antiprion activity was based on western blot densitometry of PrPSc and it was expressed as the average percentage reduction of PrPSc in compound-treated cells compared with untreated controls.
Table 1. Efficacy and cellular toxicity screen of 1,4-benzoxazine compounds for PrPSc inhibition in ScN2a cells
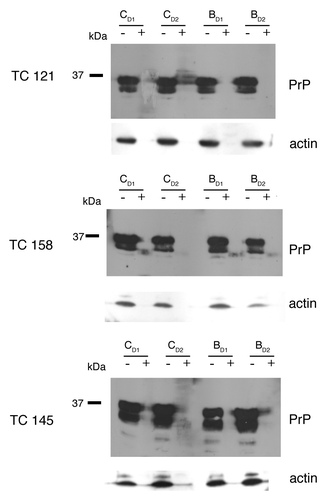
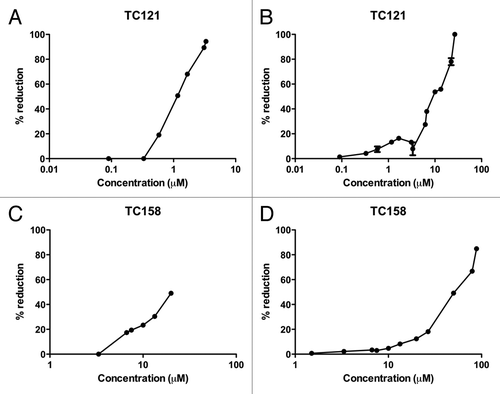
Compounds TC121 and TC158 were shown to reduce PrPSc levels in ScN2a cells, as derived by western blot analysis (). These effects were visible 4 d after the beginning of treatment. Treatment of the cells with TC158 resulted in a moderate (50%) PrPSc reduction in comparison to the impressive 90% reduction after TC121 administration. The EC50 concentration of TC121, at which 50% of PrPSc production is eliminated, was 1.17 μΜ and the EC90 was 3.2 μΜ (). Compound TC145 was similarly tested resulting in no detectable decrease of PrPSc accumulation (). Compounds TC128, TC143 and TC161 failed to produce a significant reduction of PrPSc accumulation (data not shown).
Figure 2. 22L-ScN2a cells. Effect of 5,7,8-trimethyl-1,4-benzoxazine derivatives on PrPSc levels by western blot analysis. (A) inhibitors of PrPSc formation. (B) compound TC145 with no inhibitory effects on PrPSc accumulation. Following incubation with each compound, the cells were treated (+) or not treated (-) with PK. All treatments were performed in duplicates (CD1, CD2 for the control, untreated cells and BD1, BD2 for the 5,7,8-trimethyl-1,4-benzoxazines -treated cells). Two representative blots for each active compound, corresponding to different cell passages, are presented in order to show the reproducibility of the acquired results. For PrP-immunostaining, 6H4 was used, whereas for signal normalization, blots were probed with β-actin (C4) antibody.
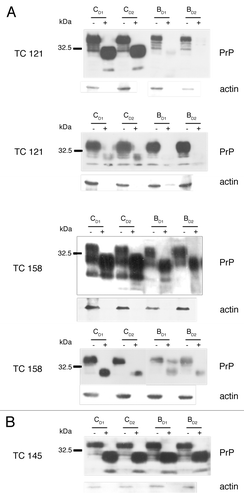
Figure 3. Dose response and toxicity curves. EC50 value, were 50% of the pathological isoform PrPSc is reduced, is 1.17 μM for TC121 (A) and 20 μM for TC158 (C). LD50 value, as derived from the representative toxicity curves, is 10 μM for TC121 (B) and 50 μM for TC158 (D).
In parallel, in order to determine whether PrPSc re-accumulates after the compound is removed from the infected cultures, cells were treated with the compounds as described in the Materials and Methods section. The fifth day after subpassaging the cells were split in a ratio 1/30. Cells were grown without treatment, lysed on the fourth day and the lysates analyzed by western blot. The analysis revealed detectable levels of PrPSc, suggesting that the effect of the studied compounds was reversible. Such a result is expected since the reduction of PrPSc signal upon treatment with the benzoxazines, albeit high (90%) is not complete, allowing the remaining PrPSc to drive generation of new PrPSc molecules in the subpassaged cells.
To evaluate the effect of the tested compounds on PrPC levels, we treated uninfected N2a cells with the compounds, using the same dosage scheme. Cytometry and Protein quantification/Densitometry were performed in order to normalize the protein levels between control and treated cells. As shown in , treatment of the cells with the active compounds TC121 and TC158 does not affect the PrPC levels in the N2a cells.
qPCR
Real time PCR experiments demonstrate a roughly 4 fold overexpression of the PRNP gene when ScN2a cells grow in the presence of 1,4-benzoxazine derivatives TC121 and TC145 as compared with untreated cells (). In contrast, treatment with compound TC158 failed to produce a significant upregulation of the PRNP transcription levels.
Figure 5. (A) Relative expression of the PRNP gene in untreated “control Sc N2a” cells and cells treated with different 5,7,8,-trimethyl-1,4-benzoxazine derivatives. (B) Relative expression of the PRNP gene in untreated “control N2a” cells and cells treated with TC121 and TC158. As expected, there is no signal in negative control samples without cDNA. (C) 2% agarose gel showing representatively the amplified fragments of the tested genes. M: relative molecular mass markers, 1, 3, 5, 7: PCR products amplified with the β-actin, PrP, RPL32 and GAPDH primers in the presence of cDNA and 2, 4, 6, 8: in the absence of cDNA.
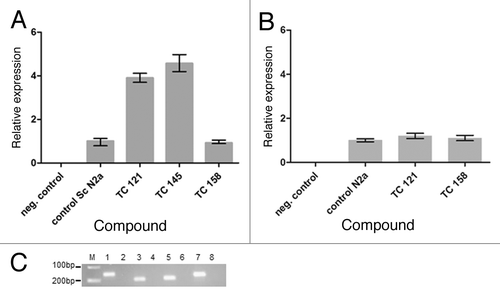
Real time PCR experiments were also performed in uninfected N2a cells following treatment with the benzoxazine derivatives with the same dosage scheme as ScN2a cells. In this case, there was no difference in PRNP gene expression levels between 1,4-benzoxazine-treated and untreated cells ().
Discussion
Cultured cell lines infected with prions are widely used to study prion conversion, as well as to screen for molecules with therapeutic potential.Citation25 The scrapie-infected N2a assay is a valuable initial screening tool for evaluating potential drugs as numerous compounds that have been identified as PrPSc inhibitors in these cells were subsequently shown to have at least some anti-scrapie activity in vivo. The mechanism, by which these compounds inhibit PrPSc formation and/or enhance its clearance, remains unknown.
Our data indicate that two out of six 5,7,8-trimethyl-1,4-benzoxazine derivatives tested, exert a diminishing effect on the levels of PrPSc. More specifically, treatment of the 22L-ScN2a cells with TC121 and TC158 reduces PrPSc accumulation by about 90% and 50% respectively, as evaluated by western blot analysis. Conversely, the other derivatives tested had no obvious effect on the levels of the pathological prion isoform. This effect appears to be PrPSc-specific, since treatment of N2a cells with the same compounds produced no difference in PrPC levels between treated and untreated (control) samples.
Even though the number of compounds examined in the present study is relatively small, we can make some preliminary structure-activity relationship observations. The effect of C6 halogen substitution on the activity of the respective compounds TC145 and TC158 is worth noting. More specifically, the bromo-substituted derivative TC145 is devoid of activity against the pathological prion isoform, while switching to a chloro group on C6 renders the respective compound TC158 active. The 4-methoxyphenyl substituent at C2 confers to the corresponding derivative TC121 high anti-prion activity (ΕC50 = 1.17 μM) and relatively low toxicity (LD50 = 10 μM). Conversely, the same substituent at C3 or N4, compounds TC161 and TC128, respectively, abolishes activity. The same effect is observed for derivative TC143 which bears a 3-methoxyphenyl substituent at C3. Based on qPCR and western blot studies in N2a cells, 1,4-benzoxazine treatment does not affect PRNP gene expression, indicating that the effective compounds TC121 and TC158 do not affect the levels of the physiological PrP protein in N2a cells. However, according to qPCR experiments in ScN2a cells, TC121 has been shown to be active at the transcriptional level as well, increasing the relative expression of the PRNP gene by a factor of 3.9 as compared with untreated cells (). Similarly, TC145 increases the mRNA levels of PrP, while the signal detected by western blot was the same as compared with control, untreated samples.
Overall, there was no correlation between the mRNA and protein data, in ScN2a cells. This is not surprising as many studies have shown that there is not always agreement between mRNA and protein levels under certain conditions.Citation26 Transcription and translation are not characterized by a linear relationship and different events may influence the dynamic properties of either transcription or translation into protein. Different mechanisms can repress the synthesis of proteins from a certain copy number of mRNA molecules. Many parameters may influence mRNA and protein correlation, such as regulatory proteins and RNA editing.Citation27 Numerous regulatory proteins can act as transcription factors. Compounds TC121 and TC145 may act directly as transcription factors of the PRNP gene or indirectly by affecting a transcription factor or other regulatory protein. An explanation for the contrast between mRNA expression and low protein levels could be the degradation of mRNA, which is widely accepted.Citation28 Different mechanisms may influence the stability of mRNA and affect translation efficiency.Citation27,Citation29
Our results indicate that TC121 affects the PrP molecule by both major pathways, at a transcriptional and the protein processing level. Further studies concerning the mechanism of action of this derivative could focus on these two levels.
The ability of the derivatives of the present study to cross the blood-brain barrier has not been determined experimentally, however calculation of the QPlogBB using QikProp v3.4, resulted in values in the range of 95% of drugs (QPlogBB for TC121 = 0.381 for TC145 = 0.576 and for TC158 = 0.570; QPlogBB range for 95% of drugs -3.0/1.2). Therefore, they may be employed as prophylactic factors against peripheral infections. Compound TC121, 2-(4-methoxyphenyl)-5,7,8-trimethyl-3,4-dihydro-2H-1,4-benzoxazine represents a lead structure requiring further derivatisation to obtain more robust structure-activity relationship data, as well as, in vivo experiments in mice to further analyze the anti-prion properties of this class of compounds and to evaluate the therapeutic potential of these 5,7,8-trimethyl-1,4-benzoxazine derivatives.
Materials and Methods
Equipment and reagents
22L-ScN2a and N2a cells that were used had been generously provided by Dr. H. Schätzl, Institute of Virology, Technical University of Munich, Germany. Handling of infectious material was performed in a safety level 2 facility.
Unless otherwise noted, all materials were obtained from commercial suppliers and used without purification. Proteinase K was purchased from Merck (Cat. Nr. 1.24569.0100). All chemicals were purchased from Sigma Aldrich. Minimal essential medium (OptiMEM® Cat. Nr. 51985042), Dulbecco’s Phosphate-Buffered Saline (DPBS Cat. Nr. 14190169), trypsin-EDTA (Cat. Nr. 25200056) and Fetal Bovine Serum (FBS Cat. Nr. 10270106) were purchased from Invitrogen-Gibco. Monoclonal anti-PrP antibody 6H4 was a generous gift from Prionics (Cat. Nr. 01–011) and monoclonal anti-actin antibody β-actin (C4) was obtained from Santa Cruz Biotechnology (Cat. Nr. 47778). Goat-anti-mouse IgG (Fc specific)-Peroxidase antibody was purchased from Sigma Aldrich (Cat. Nr. A0168), Alkaline Phosphatase (AP) conjugated Rabbit anti-Mouse IgG (Cat. Nr. 31329) and Enhanced Chemiluminescence (ECL Cat. Nr. 32106) were purchased from Pierce, while CD Pstar western blotting substrate was from NEB (Cat. Nr. 870115). iScriptTM cDNA synthesis kit was purchased from Biorad Laboratories (Cat. Nr. 170–8890) and KAPA SYBR fast qPCR kit from KAPA Biosystems (KK4601).
Synthesis of the compounds
Compounds TC121, TC145 and TC158 were synthesized as previously reported.Citation30,Citation31 The synthesis of compounds TC143, TC128, TC161 will be reported elsewhere.
Cell culture
Cells were cultivated as previously described.Citation24 More specifically, 22L-ScN2a and N2a cells were cultured in Opti-MEM+ (Opti-MEM, 10% FCS, 1% P/S) in 5% CO2 at 37°C. The medium was replaced every three days and subpassaging of the cells was performed every five days for the 22L-ScN2a and every four days for the N2a cells.
Compound preparation and incubation
Compounds were diluted in dimethyl sulfoxide (DMSO), tested in several concentrations of the drug. Final DMSO concentration did not exceed 0.5% v/v and control cell cultures were treated with DMSO only, in concentrations equal to the final concentration of DMSO delivered with the compound in each plate. To minimize the effect of variables throughout the culture and subpassaging, each compound concentration was tested in duplicates in at least three independent experiments, using cells derived from different passages, covering passage 8 to passage 15. PrPSc levels do not vary among the passages used. The compounds were administered every day for three days on equal amounts, starting on the first day after the split. TC121 was administered as following: 1st day 3.33μΜ, 2nd day 6.66 μΜ and 3rd day 3.33μΜ. For TC158 the dosage scheme included: 1st day 20μΜ, 2nd day 40μΜ and 3rd day 20μΜ. These concentrations refer to the final concentration of the compounds tested in the medium. Since preliminary observations indicate that the compounds are stable in the culture conditions, concentrations of the compounds after the second administration are temporary doubled due to the protocol requirements for cell growth. This increase didn’t influence the final effect as it could be judged when the titrations for efficacy and cellular toxicity performed and the medium was changed daily (). Cells were lysed on the fourth day after subpassaging, when, according to our pilot studies, PrPSc levels can be detected by western blotting for the first time.
Trypsinization, lysis and proteinase K treatment
For trypsin treatment, intact cells were rinsed once in PBS and then incubated with 0.25% w/v trypsin for 2 min at room temperature. Cells growing in a 100 x 20 mm polystyrene cell culture dish (Corning Cat. Nr. 430167) were split to a ratio of 1:30 into 60 x 15 mm cell culture dishes (Corning Cat. Nr. 430166). For lysis, they were rinsed once with PBS and then incubated with ice-cold lysis buffer, containing 10 mM Tris (pH 7.5), 100mM NaCl, 10 mM EDTA, 0.5% Triton-X-100 v/v, 0.5% sodium deoxycholate w/v, for 10 min at room temperature. The lysed cells were then centrifuged for 1 min at 14000 g to remove the cell debris. Half of each supernatant was methanol precipitated at -80°C for at least 2 h following the addition of PMSF to achieve a final concentration of 5mM. This sample was used to determine total PrP content in the cell lysate. The remaining lysate was treated with PK to digest PrPC and allow detection of the PK-resistant PrPSc present. For PK-treatment, cell lysates were incubated with PK (1 μg/ml) for 1 h at 37°C and then the digestion was stopped by the addition of PMSF to a final concentration of 5 mM. Finally, lauryl-sarcosyl 1% w/v was added and the proteins were methanol precipitated at -80°C for at least 2 h. Following centrifugation at 1700 g for 40 min, the protein pellets were briefly washed in 25mM Tris (pH 8.8) containing 0.05% w/v lauryl-sarcosyl and then centrifuged at 1700 g for 10 min. All pellets were resuspended in 2.5× O’ Farrell loading buffer, boiled for 10 min, briefly centrifuged and finally analyzed by SDS-PAGE and western blotting.
Cytometry
Immediately before cell lysis, cells of each plate were checked for toxic effects, bacterial contamination and density by light microscopy. Any differences in the cells compared with controls were noted. Under the conditions of growth from low density to confluence in the presence of the tested compounds, cytotoxicity is usually obvious by light microscopy. However, cytometry was performed on a microscope Neubauer chamber following staining with trypan blue. More specifically, the fourth day post subpassaging, trypsinization of the cells was performed and 50μl of the detached cells were diluted in equal volume of trypan blue. 10μl of the mixture were loaded on the Neubauer chamber and viable cells were measured using a light microscope. The trypsinized cells were then centrifuged at 1700 g for 10 min. The pellets were resuspended in 1 ml ice-cold lysis buffer and incubated for 10 min at RT, as described in the section Trypsinization, lysis and proteinase K treatment. The acquired values were plotted in order to determine the LD50 values ().
SDS-PAGE and antibody blotting
Protein samples were separated by SDS-PAGE on 12% w/v polyacrylamide gels. Equal loads for SDS-PAGE analysis were estimated according to the cytometry results. In the case of PK treated samples, the protein amount loaded on each well was four times higher as compared with non PK - treated samples. Proteins were then transferred to Polyvinylidene Fluoride membranes using a mini-transblot cell for 2 h at 100 V at 4°C. The PVDF membrane was blocked for 1 h at room temperature with blocking buffer [5% w/v non-fat dry milk in PBS containing 0.1% v/v Tween 20 (PBST)] and incubated overnight at 4°C with the primary antibody diluted in blocking buffer. For detection of PrP levels the monoclonal antibody used was 6H4 at a dilution 1:5000. After washing with PBST, the membrane was incubated for 1 h at room temperature with HRP- conjugated goat anti-mouse IgG (1:10000) and PrP was visualized on X-ray films by ECL. The same membrane was kept for estimation of actin levels and after washing with PBST for 10 min, it was incubated with β-actin (C4) antibody at 1:2000 for 1 h. After washing with PBST, the membrane was incubated for 1 h at room temperature with AP-conjugated rabbit anti-mouse IgG at 1:10000. Finally, the membrane was developed with CDP-star and exposed to X-ray films, as specified by the manufacturer.
Protein quantification/densitometry
Normalization of protein levels was performed based on actin levels. Protein content was estimated densitometrically using ImageJ software (version 1.37v, available at http://rsb.info.nih.gov/ij/) through the analysis of multiple film exposures to ensure that comparisons were made within the linear range of the film.
RNA isolation
Total RNA was isolated from N2a cells on the morning of the fourth day and from 22L-ScN2a cells on the evening of the fourth day post-subpassaging using Nucleospin RNA II columns. RNA elution was performed with 60 μl of RNase-free H2O followed by denaturation at 65°C for 5 min, according to the manufacturer’s instructions. The purified RNA was quantified spectrophotometrically using Nanodrop 2000, Thermo Scientific. cDNA synthesis was performed using iScript cDNA synthesis kit with 1 μg of total RNA.
qPCR
Real-time quantitative PCR (qPCR) was performed using the KAPA SYBR fast qPCR kit. One μl of cDNA corresponding to 50 ng of total RNA was used as template for each single PCR reaction. Reliable results were obtained with a normalization factor based on three reference genes (β-actin, glyceraldehyde-3-phosphate dehydrogenase and ribosomal protein L32) and the reaction was performed in triplicates for each sample. The same experiment was repeated three times and the RNA for each experiment was isolated from cells of different subcultures. The primer sequences for the amplification of gene fragments encoding murine PrP, β-actin, glyceraldehyde-3-phosphate dehydrogenase and ribosomal protein L32 gene fragments are given in . qPCR was performed in the 7500 Fast Real-time PCR system (Applied Biosystems). After initial denaturation for 2 min at 95°C, the following cycle was repeated 40 times: 3 sec at 95°C, 20 sec at 60°C and 30 sec at 72°C with a final extension of 3 min at 72°C. Accumulation of PCR product was monitored by measurement of SYBR Green I emission at the end of the extension step after each cycle. Melting curve analysis was performed by measuring the emission of SYBR Green I from 60°C to 95°C with a ramp rate of 1% and 10 sec hold at each temperature. PCR amplification products were visualized after separation on a 2% TBE agarose gel subsequently stained with ethidium bromide.
Table 2. Primer sequences used for the amplification of fragments from genes encoding murine PrP (PRNP) and three reference proteins: β-actin (Actb), glyceraldehyde-3-phosphate dehydrogenase (Gapdh), ribosomal protein L32 (Rpl32)
| Abbreviations: | ||
| TSEs | = | transmissible spongiform encephalopathies |
| PK | = | proteinase K |
| ScN2a | = | scrapie-infected neuroblastoma cells |
| LD50 | = | 50% lethal dose |
| DMSO | = | dimethyl sulfoxide |
| PVDF | = | polyvinylidene fluoride |
| GAPDH | = | glyceraldehyde-3-phosphate dehydrogenase |
| Rpl32 | = | ribosomal protein L32 |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr G. Mosialos for making the cell culture facility available. We also like to thank Dr C. Panagiotidis for her critical reading and suggestions she made for the manuscript.
In memory of the colleague Dr Isabel Minguez Tudela for her generous contribution in the field of Prion Diseases
References
- Prusiner SB. Shattuck lecture--neurodegenerative diseases and prions. N Engl J Med 2001; 344:1516 - 26; http://dx.doi.org/10.1056/NEJM200105173442006; PMID: 11357156
- Prusiner SB. Prions. Proc Natl Acad Sci U S A 1998; 95:13363 - 83; http://dx.doi.org/10.1073/pnas.95.23.13363; PMID: 9811807
- Aguzzi A, Glatzel M, Montrasio F, Prinz M, Heppner FL. Interventional strategies against prion diseases. Nat Rev Neurosci 2001; 2:745 - 9; http://dx.doi.org/10.1038/35094590; PMID: 11584312
- Cashman NR, Caughey B. Prion diseases--close to effective therapy?. Nat Rev Drug Discov 2004; 3:874 - 84; http://dx.doi.org/10.1038/nrd1525; PMID: 15459678
- Solassol J, Crozet C, Lehmann S. Prion propagation in cultured cells. Br Med Bull 2003; 66:87 - 97; http://dx.doi.org/10.1093/bmb/66.1.87; PMID: 14522851
- Béranger F, Mangé A, Solassol J, Lehmann S. Cell culture models of transmissible spongiform encephalopathies. Biochem Biophys Res Commun 2001; 289:311 - 6; http://dx.doi.org/10.1006/bbrc.2001.5941; PMID: 11716473
- Trevitt CR, Collinge J. A systematic review of prion therapeutics in experimental models. Brain 2006; 129:2241 - 65; http://dx.doi.org/10.1093/brain/awl150; PMID: 16816391
- Gilch S, Nunziante M, Ertmer A, Schätzl HM. Strategies for eliminating PrP(c) as substrate for prion conversion and for enhancing PrP(Sc) degradation. Vet Microbiol 2007; 123:377 - 86; http://dx.doi.org/10.1016/j.vetmic.2007.04.006; PMID: 17493775
- Caughey B, Raymond GJ. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol 1993; 67:643 - 50; PMID: 7678300
- Yudovin-Farber I, Azzam T, Metzer E, Taraboulos A, Domb AJ. Cationic polysaccharides as antiprion agents. J Med Chem 2005; 48:1414 - 20; http://dx.doi.org/10.1021/jm049378o; PMID: 15743185
- Mangé A, Nishida N, Milhavet O, McMahon HE, Casanova D, Lehmann S. Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected cultures. J Virol 2000; 74:3135 - 40; http://dx.doi.org/10.1128/JVI.74.7.3135-3140.2000; PMID: 10708429
- Pocchiari M, Schmittinger S, Masullo C. Amphotericin B delays the incubation period of scrapie in intracerebrally inoculated hamsters. J Gen Virol 1987; 68:219 - 23; http://dx.doi.org/10.1099/0022-1317-68-1-219; PMID: 2433387
- Rudyk H, Vasiljevic S, Hennion RM, Birkett CR, Hope J, Gilbert IH. Screening Congo Red and its analogues for their ability to prevent the formation of PrP-res in scrapie-infected cells. J Gen Virol 2000; 81:1155 - 64; PMID: 10725446
- Supattapone S, Nishina K, Rees JR. Pharmacological approaches to prion research. Biochem Pharmacol 2002; 63:1383 - 8; http://dx.doi.org/10.1016/S0006-2952(02)00874-2; PMID: 11996878
- Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci U S A 2001; 98:9836 - 41; http://dx.doi.org/10.1073/pnas.161274798; PMID: 11504948
- Kocisko DA, Baron GS, Rubenstein R, Chen J, Kuizon S, Caughey B. New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J Virol 2003; 77:10288 - 94; http://dx.doi.org/10.1128/JVI.77.19.10288-10294.2003; PMID: 12970413
- Kocisko DA, Morrey JD, Race RE, Chen J, Caughey B. Evaluation of new cell culture inhibitors of protease-resistant prion protein against scrapie infection in mice. J Gen Virol 2004; 85:2479 - 83; http://dx.doi.org/10.1099/vir.0.80082-0; PMID: 15269390
- Thompson MJ, Borsenberger V, Louth JC, Judd KE, Chen B. Design, synthesis, and structure-activity relationship of indole-3-glyoxylamide libraries possessing highly potent activity in a cell line model of prion disease. J Med Chem 2009; 52:7503 - 11; http://dx.doi.org/10.1021/jm900920x; PMID: 19842664
- Kawasaki Y, Kawagoe K, Chen CJ, Teruya K, Sakasegawa Y, Doh-ura K. Orally administered amyloidophilic compound is effective in prolonging the incubation periods of animals cerebrally infected with prion diseases in a prion strain-dependent manner. J Virol 2007; 81:12889 - 98; http://dx.doi.org/10.1128/JVI.01563-07; PMID: 17881452
- Ghaemmaghami S, May BC, Renslo AR, Prusiner SB. Discovery of 2-aminothiazoles as potent antiprion compounds. J Virol 2010; 84:3408 - 12; http://dx.doi.org/10.1128/JVI.02145-09; PMID: 20032192
- Wang L, Ankati H, Akubathini SK, Balderamos M, Storey CA, Patel AV, et al. Identification of novel 1,4-benzoxazine compounds that are protective in tissue culture and in vivo models of neurodegeneration. J Neurosci Res 2010; 88:1970 - 84; PMID: 20143421
- Koini EN, Papazafiri P, Vassilopoulos A, Koufaki M, Horváth Z, Koncz I, et al. 5,7,8-Trimethyl-benzopyran and 5,7,8-trimethyl-1,4-benzoxazine aminoamide derivatives as novel antiarrhythmics against ischemia-reperfusion injury. J Med Chem 2009; 52:2328 - 40; http://dx.doi.org/10.1021/jm801228h; PMID: 19309156
- Race RE, Fadness LH, Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol 1987; 68:1391 - 9; http://dx.doi.org/10.1099/0022-1317-68-5-1391; PMID: 3106566
- Nishida N, Harris DA, Vilette D, Laude H, Frobert Y, Grassi J, et al. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J Virol 2000; 74:320 - 5; http://dx.doi.org/10.1128/JVI.74.1.320-325.2000; PMID: 10590120
- Vilette D. Cell models of prion infection. Vet Res 2008; 39:10; http://dx.doi.org/10.1051/vetres:2007049; PMID: 18073097
- Gao H, Frost MR, Siegwart JT Jr., Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis 2011; 17:903 - 19; PMID: 21541268
- Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett 2009; 583:3966 - 73; http://dx.doi.org/10.1016/j.febslet.2009.10.036; PMID: 19850042
- Schedel J, Distler O, Woenckhaus M, Gay RE, Simmen B, Michel BA, et al. Discrepancy between mRNA and protein expression of tumour suppressor maspin in synovial tissue may contribute to synovial hyperplasia in rheumatoid arthritis. Ann Rheum Dis 2004; 63:1205 - 11; http://dx.doi.org/10.1136/ard.2003.006312; PMID: 15361372
- MacKay VL, Li X, Flory MR, Turcott E, Law GL, Serikawa KA, et al. Gene expression analyzed by high-resolution state array analysis and quantitative proteomics: response of yeast to mating pheromone. Mol Cell Proteomics 2004; 3:478 - 89; http://dx.doi.org/10.1074/mcp.M300129-MCP200; PMID: 14766929
- Koini EN, Avlonitis N, Calogeropoulou T. Simple and Efficient Method for the Halogenation of Oxygenated Aromatic Compounds. Synlett 2011; 1537 - 42
- Filippou PS, Koini EN, Calogeropoulou T, Kalliakmani P, Panagiotidis CA, Kyriakidis DA. Regulation of the Escherichia coli AtoSC two component system by synthetic biologically active 5;7;8-trimethyl-1;4-benzoxazine analogues. Bioorg Med Chem 2011; 19:5061 - 70; http://dx.doi.org/10.1016/j.bmc.2011.06.029; PMID: 21757361