Abstract
Cellular prion protein (PrPC) has attracted considerable attention for its role in transmissible spongiform encephalopathies (TSEs). In spite of being a point of intense research effort critical questions still remain regarding the physiological function of PrPC and how these functions may change with the conversion of the protein into the infectious and pathological conformation (PrPSc). While emerging evidence suggests PrPC/Sc are involved in signal transduction there is little consensus on the signaling pathways associated with the normal and diseased states. The purported involvement of PrPC in signal transduction, and the association of TSEs with neural pathology, makes kinome analysis of human neurons an interesting and appropriate model to characterize patterns of signal transduction following activation of PrPC by two commonly employed experimental ligands; antibody-induced dimerization by 6H4 and the amino acids 106-126 PrP peptide fragment (PrP 106–126). Analysis of the induced kinome responses reveals distinct patterns of signaling activity following each treatment. Specifically, stimulation of human neurons with the 6H4 antibody results in alterations in mitogen activated protein kinase (MAPK) signaling pathways while the 106-126 peptide activates growth factor related signaling pathways including vascular endothelial growth factor (VEGF) signaling and the phosphoinositide-3 kinase (PI3K) pathway. These pathways were validated through independent functional assays. Collectively these results indicate that stimulation of PrPC with distinct ligands, even within the same cell type, results in unique patterns of signaling. While this investigation highlights the apparent functional versatility of PrPC as a signaling molecule and may offer insight into cellular mechanisms of TSE pathology it also emphasizes the potential dangers associated with attributing activation of specific intracellular events to particular receptors through artificial models of receptor activation.
Introduction
Transmissible spongiform encephalopathies (TSEs) include Creutzfeldt-Jakob disease in humans, bovine spongiform encephalopathy in cattle, scrapie in sheep and chronic wasting disease in deer and elk. These diseases represent the first characterized example of an infectious disease which is mediated exclusively by a protein agent.Citation1 TSEs result from the autocatalytic conversion of endogenously expressed cellular prion protein (PrPC) to an infectious conformation; the scrapie like variant (PrPSc). Due to the central role of PrPC in these fatal neurodegenerative disorders it has prompted extensive investigations of the biology of this widely expressed, highly conserved protein. In spite of these efforts fundamental questions about the physiological and pathophysiological roles of the protein remain. Specifically, the physiological role of PrPC, and the mechanisms by which PrPSc mediates disease pathology, remains unclear. A central challenge to understanding the molecular basis of TSEs is determining whether the conformational conversion of PrPC to PrPSc represents a gain of function, loss of function or change of function.
Efforts to elucidate the function of PrPC through deletion have been largely uninformative as, other than resistance to prion diseases, the phenotype associated with PrPC−/− in mice is quite mild.Citation2 This has required researchers to adopt more subtle, but often necessarily biased, investigations of PrPC function. From these efforts a variety of physiological functions of PrPC have been suggested including: neural protection,Citation3 copper metabolism,Citation4 long-term memory formation,Citation5 and bone marrow renewal.Citation6 There is equal uncertainty of the mechanism of PrPSc pathology.
That PrPC is a member of the glycophosphatidylinositol (GPI) anchored proteins which suggests a potential role in signal transduction. GPI anchored proteins are characteristically localized to lipid rafts which are functional hubs of signal transduction activity.Citation7 The contribution of GPI-anchored proteins to signal transduction is often through interaction with other transmembrane signaling proteins.Citation8 Furthermore, the pathology of PrPSc appears to be dependent on its association with the lipid rafts; a soluble version of the protein, created through removal of the GPI-anchor, retains the ability to form plaques but without producing classical prion disease.Citation9 While subsequent studies showed that anchorless PrP expressed at 2-fold greater levels and exposed to PrPSc will result in disease, this disease displays many distinct characteristics from classical, GPI-anchored prion disease.Citation10,Citation11 This observation is essential in separating protein aggregation from disease pathology and suggests the deleterious effects of TSEs are associated with a change in information transmitted across the membrane. Specifically, the dual requirement for conversion to PrPSc, as well as the association with lipid rafts suggest a subversion of the normal function of PrPC upon misfolding to PrPSc that involves distortion of signaling events.Citation12
Subsequent investigations of PrPC and PrPSc from the perspective of signaling analysis have implicated a number of intracellular kinases and signaling pathways including: Sarcoma (Src),Citation13 mitogen activated protein kinase (MAPK),Citation14,Citation15 phosphoinositide-3 kinase (PI3K)/ RAC-α series/threonine protein kinase (Akt),Citation16 c-Jun N-terminal kinase (JNK),Citation17 and extracellular signal-regulated kinase (Erk).Citation18 While these investigations strongly indicate that PrP influences the intracellular kinase environment there is no consensus on the specific mechanism and consequences. The diversity of signaling events ascribed to PrPC/Sc is a likely a partial consequence of the unavoidable bias, and limited scope, of various investigations but may also reflect the biological complexity of this protein. In particular, variables such as species, cell type, conformational isomer and, in the absence of a defined ligand, different experimental models of PrPC/Sc activation may contribute to the diversity of signaling pathways which have been reported to date.
In the current study we investigate patterns of signal transduction in human neuronal cells in response to activation of PrPC by two commonly employed experimental ligands; antibody-mediated dimerization by 6H4, which has been suggested as a model for activation of PrPC 19 as well as the amino acids 106-126 peptide fragment of human PrPC (PrP 106–126), which has been proposed as an experimental model of PrPSc like activity.Citation20 The PrP 106-126 peptide fragment has been widely utilized as an experimental ligand to model prion effects and this made it an ideal stimulant of PrPC signaling.Citation17,Citation20-Citation24 In addition, 6H4 is a well-characterized, commercially-available monoclonal antibody for PrPC. Prion protein specific antibody has been utilized to “activate” PrPC through direct binding for investigations of PrPC function.Citation19
Analysis is performed through peptide arrays which offer a more global and unbiased representation of signaling events than other methods. Cluster analysis of the kinome data reveals highly distinct patterns of signaling activity under the different treatment conditions. This is supported by pathway analysis which indicates the 6H4 antibody treatment results in alteration of MAPK signaling pathways whereas the 106-126 treatment is primarily associated with alteration of growth factor receptor and PI3K signaling. These results indicate activation of distinct patterns of signaling through PrPC in the same cell type under different treatment conditions. This supports the hypothesis of the functional versatility of PrPC as a signaling-associated molecule and may offer insight into the mechanism of TSEs.
Results
Cell surface expression of the PrPC protein
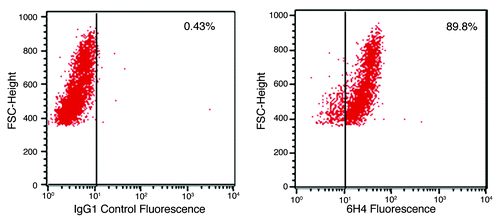
As this study is concerned with the stimulation of PrPC on the surface of cells it was necessary to confirm cell surface expression of the protein. Flow cytometric analysis of the human neuronal cell line confirmed high levels of PrPC expression, as indicated by 6H4 binding, relative to an irrelevant IgG1 isotype control antibody ().
Figure 1. PrP surface expression. Shown are flow cytometic analyses plots of neuronal cells labeled with antibody 6H4. A secondary antibody, GAM-FITC, was used to visualize the cells. The plots to the left shows non-specific GAM-FITC binding following incubation with an irrelevant isotype control IgG antibody. The plot to the right depicts cell fluorescence following incubation with the PrP-specific antibody 6H4 and the FITC-conjugated GAM. The % shows the percent of cells which have shifted, indicating fluorescence. Media control cell autofluorescence (no antibody added) resulted in 1.05% of cells indicating fluorescence (data not shown).
Peptide array kinome analysis of treated cells
Previously we have demonstrated the utility of peptide arrays for the characterization of signaling events associated with activation of specific receptors.Citation25,Citation26 This included descriptions of previously uncharacterized signaling events following activation of specific receptors with defined ligands. Here a similar approach is adopted to define signaling events associated with the two distinct experimental models of PrPC activation.
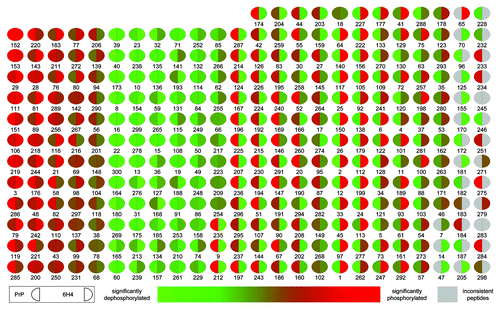
The array developed within our lab consists of 300 unique peptides, each printed in triplicate, representing key phosphorylation events from a spectrum of central signaling pathways and cellular processes. A display of relative signal for each array peptide under the treatment conditions clearly illustrate that the different methods of PrPC activation result in distinct patterns of intracellular kinase activity (). These differential responses are highly conserved across the biological and technical replicates. In the case of peptide stimulation, 116 out of 300 displayed a significant and conserved response while for the 6H4 antibody stimulation 120 out of 300 peptides displayed a significant and conserved pattern of differential responses across the biological and technical replicates. This strong conservation of signal between replicates indicates an ability to detect and quantify dynamic patterns of signaling within each treatment condition. The complete peptide array data sets for the two treatments are included in Table S1.
Figure 2. Peptide array pseudo-image. Shown is a pseudo-image of a peptide array block combining the prion protein stimulation data. Transformed data for PrP 106-126 is compared with scramble control peptide data, and a significance value is determined based on a significant increase or decrease in phosphorylation between the two. This process is also performed for 6H4 antibody stimulated and isotype control antibody. The relative intensity of each spot indicates the level of significance of the phosphorylation of that spot. The left side of each spot is the response to PrP 106-126 stimulation. The right side of each spot indicates 6H4 stimulation response. Grey spots represent inconsistent peptides between array peptide replicates as determine by Χ2 test
Cluster analysis of kinome data
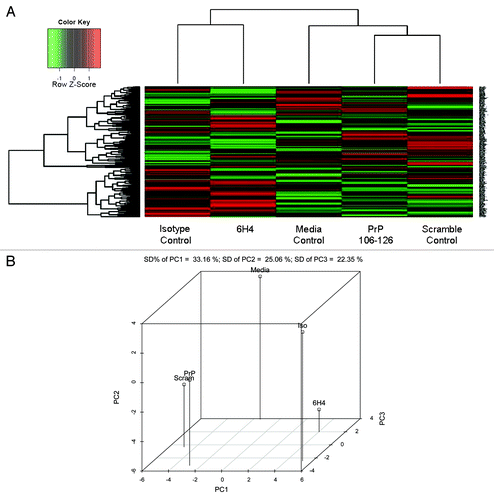
Cluster analysis of the kinome data sets was performed for comparative visualization of the kinome responses under the various treatment conditions. Cluster analysis without subtraction of the corresponding biological controls demonstrates that the data sets emerging from treatment with antibody (whether 6H4 or isotype control) cluster differently from the cells treated with peptide (whether 106-126 or the scrambled control peptide) (). This is further supported by 3D Principal Component Analysis () which indicates that both PrP 106-126 and scramble control peptide cluster close together on three of three axes. While 6H4 and antibody control cluster more closely on one of three axes it is the principal component one (PC1) which accounts for the greatest amount of variation. The clustering pattern of each treatment condition, as well as distinct patterns evident in the visual representation of the data sets, empathizes the power of the arrays in detecting subtle changes in biology as well as the importance of selecting the appropriate biological controls. Clustering suggests following treatment with the specificity controls there are non-specific effects occurring, peptide signal and antibody signal, which is not specific to prion protein function. Improper selection of biological controls, such as using media control as the control for either the 6H4 or 106-126 peptide treatment, may suggest signaling events which are not specific to PrPC and could contribute to reported inconsistencies in PrPC signaling activities. Thus the clustering allows us to see that there are non-specific effects and allows us to correct for them in the most appropriate way, by utilizing the appropriate specificity control to eliminate any non-specific signal. This correction was done for all subsequent analysis.
Figure 3. Phosphorylation Heat Map and Clustering. (A) The background-corrected raw data collected from the peptide arrays were VSN-transformed, and a heatmap/clustering of the data was produced using the Complete Linkage + Euclidian Distance method. The lines at the top of the heatmap indicate the relative similarity between the stimulants indicated at the bottom of the heatmap. The shorter the lines, the more similar the two connected stimulants. The lines on the left side of the heatmap indicate the relative similarity in signal between the 300 individual peptides on the array. The colored lines indicate the relative degree of phosphorylation of each peptide from strongly phosphorylated (red) to non-phosphorylated (green) as indicated by a Z-score. (B) Shown here is the 3D Principal Component Analysis of the five treatments. Relative distance on the three axes indicates level of similarity or difference among the treatments. PrP refers to PrP 106-126 peptide. Scram refers to scramble control peptide. 6H4 refers to the PrP-specific antibody. Iso refers to the IgG1 isotype control antibody. Media refers to media control.
Unique patterns of signaling with PrP 106-126 and 6H4 PrPC stimulation
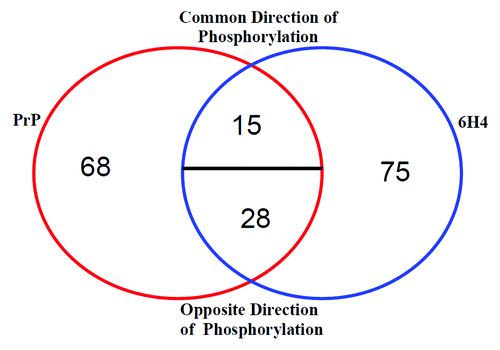
To illustrate the difference in phosphorylation pattern following the treatments, a Venn diagram of the differentially phosphorylated peptides is shown (). The figure indicates that between the two treatments 186 out of 300 peptides on the array show significant differential phosphorylation. Of these 68 peptides are observed only in PrP 106-126 treated and 75 peptides are only observed following treatment with 6H4. Of the 43 peptides common between the treatments only 15 indicate phosphorylation in a common direction, either increased or decreased relative to respective control. When these 15 proteins are run through the InnateDB pathway search there is no specific response common among them (data not shown). These data indicate a substantial difference in response between the two treatments relative to control. Table S1 lists the unique peptides and their phosphorylation target amino acids for the different stimulations.
Figure 4. Stimulation comparison of peptide phosphorylation. Peptides displaying differential phosphorylation following the two stimulations are compared. The majority of the significantly differentially phosphorylated peptides are unique to one stimulant or the other. A minority of the peptides are common to both PrP 106-126 and 6H4. Common Direction of Phosphorylation refers to peptides displaying either increased or decreased phosphorylation following both stimulations. Opposite Direction of Phosphorylation refers to one stimulation resulting in an increased phosphorylation, while the other stimulation results in a decrease.
Pathway analysis of kinome data
The kinome data was subjected to pathway analysis to determine which cellular pathways/processes are activated under the different treatment conditions. To ensure the identified pathways represent conserved and consistent biological responses, input data was limited to peptides with a consistent pattern of phosphorylation across the technical replicates and significant changes in phosphorylation level relative to the control treatment (p < 0.20). Significant pathways (p < 0.05) for each stimulation condition are shown ().
Table 1. Innate DB generated pathway list
PrP 106–126
Growth factor signaling by 106-126
Pathway analysis of the kinome data relating to activation of human neuronal cells with the PrP 106-126 peptide, relative to the scrambled peptide, indicates activation of pathways associated with growth factor signaling. In particular, there is a high degree of confidence in activation of VEGF (p = 0.014) and PI3K signaling events (p = 0.017) (). VEGF has been linked to neurodegenerative diseasesCitation27 and PI3K/Akt is a crucial signaling protein in cell survival,Citation28 both closely associated with previously described potential PrPC function. A representative signaling pathway indicting the activation of VEGF and PI3K signaling, as well as points of interaction between these pathways, is presented in ().
Figure 5. Signaling pathways linked to PrPC stimulation. Shown is a selection of proteins implicated by peptide array data that is organized into interconnected pathways. Yellow indicates unique PrP 106-126-related phosphorylation. Orange indicates unique 6H4-related phosphorylation. Red indicates that both stimulants affect the peptide in the same direction of phosphorylation. Green indicates that both stimulants affect the peptide in opposite directions. Clear indicates insignificant signal or that the peptide is not present on the array.
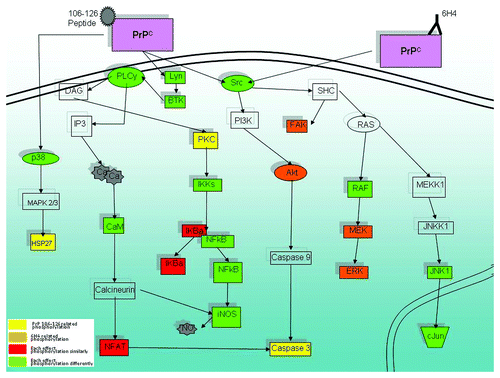
Activation of vascular endothelial growth factor receptor (VEGFR) signaling may reflect that PrPC signaling in some way interacts with the VEGFR, VEGFR signaling intermediates, or with growth factor receptors more generally. For example, fibroblast growth factor receptor (FGFR) may also interact with PrPC as it is significantly phosphorylated on the array (p = 0.05) as is nerve growth factor receptor (NGFR) (p = 0.13), and platelet derived growth factor receptor (PDGFR) β (p = 0.06).
Apoptosis signaling by 106–126
Apoptosis and its corollary cell growth and survival are among the most strongly linked functions related to the prion protein.Citation3 Previous studies have purported to describe the mechanism by which prion, and specifically PrP 106-126, regulates apoptosis.Citation22,Citation29 Prion related apoptosis studies have proposed a multitude of mechanisms, which are often contradictory, for the induction of apoptosis following prion protein stimulation.Citation3 The data reported here seems to indicate an apoptotic response via Caspase 3 (). Capase 3 appears to be regulated in two possible ways: through the Akt pathwayCitation30 and/or via the calcium pathway and nuclear factor of activated T-cells (NFAT).
Ca signaling by 106-126
Calcium signaling appears to be an important aspect of the prion protein function and its prion related role has been reported for well over a decade.Citation31 The signaling molecules observed in the peptide array provide a hypothetical pathway involving calcium signaling () via phospholipase C gamma (PLCγ), calcineurin and NFAT.Citation32
Integrin signaling by 106-126
Following PrP 106-126 stimulation the peptide array data implicated a pathway linked to integrin signaling. While conducting our analysis of the data this result was initially confusing. Many of the pathways generated from the peptide array data were results that had been observed elsewhere in the literature. There appeared to be little previous evidence implicating integrin directly to the prion protein. However, a recent study, published since this data was collected, has found a link between the prion protein’s signaling ability and neuronal cell integrin.Citation33 This recent confirmation of our results again adds evidence that our signaling observations are accurate representations of prion protein related signaling.
The signaling results from PrP 106-126 treatment, taken together with previous reports of the peptide being model of PrPSc provide some possible insights into disease state signaling. The main branches of calcium signaling, growth factor receptor and Akt/PI3K, relate directly to cell life and death. An alteration in these pathways could cause a cell to undergo apoptosis and may be the subversion of PrPC’s normally protective function that causes the loss of neuronal cells in prion disease ().
6H4
MAPK signaling and a number of related pathways are strongly implicated following 6H4 treatment (). The MAPK signaling pathway is a central pathway to many key cellular functions including cell proliferation, cell cycle, differentiation, immunity and apoptosis.Citation34 As a result it could be expected that the MAPK signaling pathway would have some function in PrPC signaling. MAPK signaling in the cell is often as a synthesizer of extracellular signaling transferring these signals to downstream effectors. It is possible that the pathway performs a similar function in PrPC signaling.
Previous prion protein studies have linked p38 MAPK to apoptosisCitation24 and inducible nitric oxide synthase (iNOS) activationCitation21 which we have observed in this study. Interestingly, neuronal cells stimulated with PrP 106-126 also show iNOS and had among the highest phosphorylation levels (at targets Y151, S909 and S739) and the highest statistical significance of any peptide studied (p values of 1.6 × 10−3, 1.6 × 10−3, and 3.1 × 10−4 respectively). So while the unique pattern of phosphorylation between the two stimulants is an important observation there are also common elements.
Insulin signaling and its related pathways appear to be a strong contender as a PrPC related receptor in 6H4 treated cells. A number of insulin related pathways appear in the analysis (). Altered insulin receptor functioning linked to PrPC in neuroblastoma cells has been observed previously.Citation35
Validation
Phosphospecific antibody
Phosphospecific antibody arrays, which quantify phosphoproteins as opposed to kinase activity, provide a distinct, yet complementary, technique to validate the phosphorylation-mediated signal transduction activity suggested by the peptide arrays. The technique is similar to using a phosphospecific antibody to perform many individual western blots simultaneously. Since the peptide array target peptides are custom designed and the phosphospecific antibodies are restricted to those commercially available the number of direct matches between the two techniques is limited. Despite this there are a number of matches between the two techniques which validate the PrP array results; these are shown in .
Table 2. Phosphorylation of select signaling molecules indicated by peptide array and phosphospecific antibody array
Both the magnitude and direction of phosphorylation tend to agree between the two techniques, providing a level of confidence in the peptide array results. The peptide array data implicated growth factor related signaling and specifically that related to VEGFR. The antibody array showed focal adhesion kinase (FAK), heat shock protein 27 (HSP27) and p38 were phosphorylated at the same sites indicated by the peptide array. These three proteins are all involved in VEGFR signaling (KEGG ID: hsa04370). Similarly calcium signaling was indicated by the peptide array and calcium/calmodulin dependent protein kinase 2 (CaMK2), a calcium related kinase (KEGG ID: hsa04020), was showed to be phosphorylated at amino acid T286 by both the peptide array and antibody array techniques. Finally, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) at S276 was shown to be phosphorylated by both techniques and is involved in apotosis signaling (KEGG ID: hsa04210). Apoptosis is a well-established effect of cell stimulation by PrP 106-126.Citation3
Viable cell count
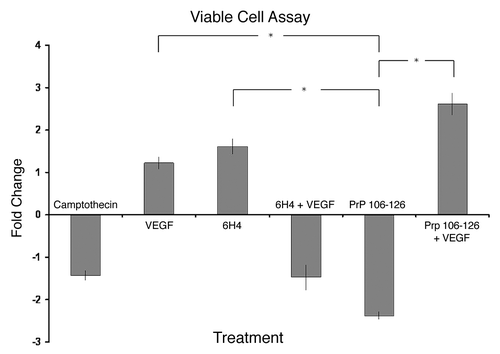
VEGF pathway disruption is an established cause of neurodegenerationCitation30 and was implicated by the array data following PrP 106-126 cell stimulation (). As mentioned previously, apoptosis-mediated cell death is a well characterized effect of PrP 106-126 treatment of cells. Considering this, it was hypothesized that the addition of recombinant VEGF protein to the cell culture may have an effect on cell death caused by PrP 106-126. shows the relative change in cell viability as compared with control under the various cell treatment conditions. The treatment with VEGF alone and 6H4 alone resulted in a slight increase in viability compared with control. The addition of VEGF and 6H4 together resulted in a slight decrease in viability. There was no statistically significant difference in the results between the 6H4 treated samples so no conclusions on 6H4 effect on viability can be drawn. As expected, PrP 106-126 resulted in a relatively large decrease in cell viability as compared with control. Most interestingly, and as hypothesized, the addition of recombinant VEGF altered the effect of PrP 106-126 on cell viability, significantly increasing viability. The results shown in also emphasize the different effects that 6H4 and PrP 106-126 appear to be having on the neuronal cells, despite their prion protein-specific nature.
Figure 6. Cell viability. Stimulated human neuronal cells were assayed for viability using trypan blue dye exclusion and a hemocytometer. Camptothecin refers to the inducer of apoptosis. PrP 106-126 refers to the prion peptide fragment. 6H4 refers to the 6H4 antibody. PrP 106-126 + VEGF refers to PrP 106-126 peptide plus the addition of recombinant VEGF. 6H4 + VEGF refers to 6H4 antibody plus recombinant VEGF. Each stimulated sample was compared with its respective control sample to keep analyses as similar to peptide array analyses as possible. Camptothecin and VEGF counts were compared with unstimulated control counts. 6H4 counts were compared with IgG1 isotype control counts. 6H4 + VEGF counts were compared with 6H4-stimulated counts. PrP 106-126 counts were compared with scramble peptide control counts. PrP 106-126 + VEGF counts were compared with PrP 106–126-stimulated counts. Assays were performed in triplicate with fold change and standard error presented. *Indicates a statistical confidence of p ≤ 0.05.
Intracellular calcium
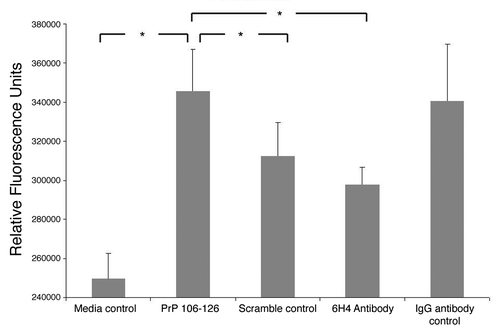
Calcium signaling has been shown in numerous studies to be important in prion protein related signaling. This was also shown by the peptide array analysis as several calcium related signaling molecules including calmodulin (CaM), NFAT2 and PLCγ showed significantly altered phosphorylation in stimulated cells (; Table S1). A intracellular calcium assay was conducted following PrPC stimulation with antibody and peptide as well as respective controls (). Results indicated a relatively large increase in intracellular calcium when cells were treated with peptide over cells treated with antibody. This result held true whether you compare peptide to antibody or peptide and antibody to their respective controls. There is a relatively large standard deviation in the IgG1 isotype control readings thus based on the statistics there is no significant difference between IgG1 control and 6H4. There is also no statistically significant difference between 6H4 and media control. Thus one cannot say with confidence that either antibody treatment has any effect on intracellular calcium levels. At the same time PrP 106-126 resulted in a statistically significant increase in intracellular calcium based on all relevant comparisons: to media control, scramble control and 6H4. These results again showed a distinct signaling event being induced by each prion protein specific treatment. These results seemed to confirm the conclusion reached from the peptide array data that peptide and antibody induce distinct signaling events in the cell through the PrPC protein.
Figure 7. Intracellular calcium. Stimulated human neuronal cells were assayed for intracellular calcium as measured by fluorescence. The assay was performed in triplicate. The means of the replicates are presented along with standard error of the means. Analysis of variance indicated a p-value of 0.032 between groups. The greater the fluorescence signal, the more intracellular calcium. Pairs which showed a statistically significant difference in signal of at least p ≤ 0.05 are indicated.
Discussion
While there have been numerous studies looking at the potential functions of PrPC and the effect on cells upon misfolding this study takes a more global perspective on the kinome changes within PrPC stimulated cells. This study points to several interesting features of PrPC signaling that have been discussed elsewhere for which we have provided increased evidence.
The fact that there are several novel and disparate pathways showing up in the analysis may indicate areas of function that have not yet been explored. They may also indicate that the normal PrPC function and the subversion of this function by misfolding into PrPSc may not affect a single pathway but several interconnected ones. shows how multiple pathways implicated by pathway analysis can be linked. The two PrPC specific treatments affect these signaling pathways in different ways indicating different stimulating ligands targeting the same surface protein can produce different signaling events within the same cell, leading to multiple possible functions.
While there is much future work to be done to provide the complete story of PrPC and prion function this study provides a number of observations and a general framework can be hypothesized. Our prion disease-like treatment using PrP 106-126 peptide displays many of the same actions as had been previously reported including PI3K, Caspase 3 and calcium response. The power of our analysis to provide a more global perspective allowed us to move up the signaling pathway to point to a putative receptor interaction with PrPC, among the most significant was the VEGF receptor. While previous prion studies have pointed to laminin receptors as possible prion protein interaction receptorsCitation36 we show that more specifically the integrin receptors may be linked to PrPC stimulation of neuronal cells, a result recently reported elsewhere.Citation33 Meanwhile, PrPC stimulation by the 6H4 antibody displayed a distinct signaling repertoire of which the strongest indicators pointed to insulin receptor/MAPK related signaling. While there is cross treatment signaling that is common to both, such as iNOS, the majority of signaling is unique to each treatment (). In addition, most of the common signaling intermediates between the two treatments are phosphorylated in opposite directions (). This unique signaling based on treatment indicates PrPC has the ability to induce differential signaling depending on ligand. Pathways such as the PI3K/calcium related pathways are observed in PrP 106-126 stimulated cells but the same pathways show no significant response when cells are stimulated with 6H4 (). Correspondingly the Erk signals as well as NFκB pathway are highly significant in 6H4 stimulated cells but completely absent following stimulation with PrP 106-126 ().
While the possible interacting partners of the PrPC protein indicated in this study are interesting more research is needed for them to be conclusively proven. The most interesting conclusion to be drawn from the given data are that PrPC protein is capable of eliciting two nearly distinct signaling events when specifically stimulated by two ligands. This variation in functional activity depending on the binding partner has not been reported before and may point to a possible reason the prion protein function has been so difficult to determine thus far. If the prion protein can signal in distinct ways depending on its ligand, possibly mechanistically through different co-receptor partners, this complicates the search for prion protein function. It may be that future studies must consider species, cell type, ligand, prion protein conformation and surface interacting partner when attributing a function to the protein because all of these may affect function.
Materials and Methods
Human neuronal cell culture
Neuronal cells were selected for investigation as they express some of the highest levels of PrPC and are of central importance in TSE pathology. Human neuronal cells BE(2)M17 (ATCC #CRL-2267 supplier Cedarlane Laboratories) were maintained in a 1:1 mixture of Eagle’s Minimum Essential medium (EMEM) (Cedarlane Laboratories) containing Earles Balanced Salt Solution, non-essential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1.5 g/L sodium bicarbonate and F12K Medium (Cedarlane) containing gentamicin (Gibco, Invitrogen) with 10% FBS (Gibco, Invitrogen). Cells were incubated at 37°C with 5% CO2 in T-75 culture flasks (Corning).
Confirmation of PrPC expression
Monoclonal antibodies, 6H4, specific for PrPC were purchased from Prionics, fluorescein isothiocyanate conjugated, isotype specific, goat anti-mouse IgG antibodies (GAM-FITC) were purchased from BD Biosciences and IgG1 control antibodies were purchased from CALTAG Laboratories. One million cells resuspended in 50 μL phosphate buffered saline (PBS) and 50 μL of 10 μg/mL primary antibody was mixed and allowed to incubate on ice for 15 min. Cells were washed three times in PBS then resuspended in 100 μL of 10 μg/mL secondary GAM-FITC and allowed to incubate for 15 min on ice. Cells were washed 3 times in PBS, resuspended in 200 μL PBS and read using a FACSCalibur (Becton Dickinson) flow cytometer with the CellQuest (Becton Dickinson) software program.
Peptide synthesis, purification and characterization
Peptides were chemically synthesized on a PIONEER solid-phase peptide synthesizer using Fmoc (9-fluorenylmethoxy carbonyl) chemistry. The peptide chains were synthesized from the carboxyl terminus to the N-terminus onto [5-(4-Fmoc-aminomethyl-3,5-dimethyloxyphenoxy) valeric acid]-polyethylene glycol-polystyrene (PAL-PEG-PS) resin. Fmoc-protecting groups at the N-terminus were deprotected with piperidine. The peptides were cleaved from the resin with concurrent deprotection of the side chain-protecting groups by treating the resin-bound peptide with trifluoroacetic acid (TFA) (9.3 parts) in the presence of scavengers (anisole-ethyl-methyl sulfide-1,2-ethanedithiol [3:3:1]), for 7 h. The crude peptides were filtered from the resin, and the TFA was evaporated. Diethyl ether was added to the residues to precipitate the crude peptide. The peptides were isolated and purified by high-performance liquid chromatography (HPLC) on VYDAC protein C4 columns (1.0 by 25 cm) eluting with a linear gradient of 10% buffer A (H2O-0.1% TFA)-90% buffer B (acetonitrile-H2O [90/10]-0.01% TFA) for 40 min at a flow rate of 3 ml/minute. Fractions of greater than 95% purity were used for the investigation. The purity and molecular weight of the respective peptides were confirmed by matrix-assisted laser desorption ionization (MALDI)-time of flight mass spectrometry on a PE Biosystems VOYAGERTM system 4068 (National Research Council, Plant Biotechnology Institute, Saskatoon, Canada).
PrP 106-126 stimulation
Prion protein fragment of amino acids 106-126 (PrP 106-126), KTNMKHMAGAAAAGAVVGGLG, corresponding to the human PrPC sequence was used to stimulate cells expressing cell surface PrPC. A peptide of identical amino acid composition but scrambled sequence NGAMAKMAGGHAVATVAGKGL was used as a peptide control. Peptides were dissolved in distilled H2O at a concentration of 5 μg/ml and added to cells in culture media to a final concentration of 80 μM, which is slightly less than that required to elicit apoptosis based on the Annexin V assay (data not shown). Cells were cultured in tissue cluster plates (Corning) and incubated at 37°C with 5% CO2.
Antibody stimulation
Antibody stimulation was done as per the cell phenotyping procedure with the following alterations. Cells were suspended in media, GAM not coupled to FITC was used as the secondary (CALTAG), 5 μg/mL of secondary was used, only 1 wash following primary addition was done, no washes after secondary addition.
Peptide arrays
Peptide array protocol was based upon a previously published protocol with modifications.Citation37 Approximately 1 X 107 cells were stimulated with PrP 106-126 at 80 µM for 4 h. Cells were collected, spun down and lysed with 100 µL lysis buffer (20 mM Tris-HCL pH 7.5, 150 mM NaCl,1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate,1 mM Na3VO4,1 mM NaF,1 µg/mL leupeptin,1 g/mL aprotinin,1 mM PMSF) (all products from Sigma Aldrich, unless indicated) was added to the cells, incubated on ice for 10 min and spun in a microcentrifuge for 10 min at 4°C. A 70 µl aliquot of this supernatant was mixed with 10 µl of the activation mix (50% Glycerol, 500 μM ATP (New England Biolabs), 60 mM MgCl2, 0.05% v/v Brij-35, 0.25 mg/mL BSA), incubated on the chip for 2 h at 37°C. Arrays were then washed in tubes containing PBS-(1%) Triton.
Slides were then submerged in phospho-specific fluorescent stain (ProQ Diamond Phosphoprotein Stain, Invitrogen) with agitation for 1 h. Arrays were washed three times for ten minutes each with destain (20% acetonitrile (EMD Biosciences) and 50 mM sodium acetate (Sigma) pH 4.0). A final wash was done with distilled H2O. Arrays were dried in 50 mL centrifuge tubes spun at 300 × g for 2 min to remove any moisture from the slides. Arrays were read using a GenePix Professional 4200A microarray scanner (MDS Analytical Technologies) at 532–560 nm with a 580 nm filter to detect dye fluorescence. Images were collected using the GenePix 6.0 software (MDS) and the spot intensity signal collected as the mean of pixel intensity using local feature background intensity background calculation with the default scanner saturation level.
For each treatment condition all stimulations were performed in triplicate for a total of nine data points for each peptide under each treatment condition (106-126 peptide, scramble control, 6H4 antibody, isotype control and media control).
Kinexus phosphospecific antibody array
Cells (1 × 107) were stimulated with 80 uM of the prion protein fragment PrP 106-126 or scramble control in media. The cells were then centrifuged at 300 × g for 8 min, washed in PBS and centrifuged a second time. The supernatant was removed and the pellet was frozen at -80°C before shipping. An antibody microarray was performed by Kinexus Bioinformatics Corp.
Viable cell count
Neuronal cells were plated in 24 well plates (Corning) at a concentration of 1 × 106 and left overnight. Cells were then treated with peptide or antibody as described previously with or without the addition of 15 ng/mL recombinant VEGF (Invitrogen). Camptothecin at a concentration of 10 μM (Sigma) was used as a positive control. Cells were left for an additional 24 h to allow stimulation to have effect and cell viability was determined by trypan blue exclusion staining and cell count using a hemocytometer.
Intracellular calcium
Cells at a concentration of 1 × 106/mL were treated with the appropriate stimulation for 4 h. Cells were harvested and resuspended at 2 × 106cells/mL in KRH buffer containing 0.1% BSA, 118 mM NaCl, 4.6 mM KCl, 24.9 mM NaHCO3, 1.0 mM KH2PO4, 11.1 mM glucose, 1.1 mM MgSO4, 5.0 mM HEPES, and 1.0 mM CaCl2 (all reagents Sigma) pH 7.4. Cells were incubated with 2 µM Fura-2/AM fluorophore at 37°C in 5% COCitation2 for 30–40 min. Cells were then centrifuged at 300 × g for 8 min resuspended in KRH buffer and incubated again for an additional 20 min at 1 × 106 cells/mL. Fluorescence was measured at 339 nm excitation and 505 nm emission.
Peptide array data analysis
Data collected from the scanned array by the GenePix software was analyzed as per Li et al.Citation38
Pathway analysis
InnateDb is a publically available resource which, based on levels of either differential expression or phosphorylation, predicts biological pathways based on experiment fold change data sets.Citation39 Pathways are assigned a probability value (p) based on the number of proteins present for a particular pathway as well as the degree to which they are differentially expressed or modified relative to a control condition. A pathway significance of p value < 0.05 was used and any pathway below this level was not considered as a possible biologically relevant pathway (). Since InnateDB requires fold-change data, the antilog of transformed intensity differences was computed and used.
| Abbreviations: | ||
| Akt | = | RAC-alpha series/threonine protein kinase |
| CaMK2 | = | calcium/calmodulin dependent protein kinase 2 |
| Erk | = | extracellular signal-regulated kinase |
| FAK | = | focal adhesion kinase |
| FGFR | = | fibroblast growth factor receptor |
| GAM-FITC | = | goat anti-mouse IgG fluorescein isothiocyanate conjugated antibodies |
| GPI | = | glycophosphatidylinositol |
| HSP27 | = | heat shock protein 27 |
| iNOS | = | inducible nitrous oxide synthase |
| JNK | = | c-Jun N-terminal kinase |
| NFAT | = | nuclear factor of actvated T-cells |
| NFκB | = | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGFR | = | nerve growth factor receptor |
| MAPK | = | mitogen activated protein kinase |
| PC1 | = | principal component one |
| PDGFR | = | platelet-derived growth factor receptor |
| PLCγ | = | phospholipase C gamma |
| PI3K | = | phosphoinositide-3 kinase |
| PrP 106-126 | = | amino acids 106-126 PrP peptide fragment |
| PrPC | = | cellular prion protein |
| PrPSc | = | scrapie-like prion protein conformation |
| Src | = | sarcoma |
| TFA | = | trifluoroacetic acid |
| TSE | = | transmissible spongiform encephalopathies |
| VEGF | = | vascular endothelial growth factor |
| VEGFR | = | endothelial growth factor receptor |
Additional material
Download Zip (195 KB)Disclosure of Potential Conflicts of Interest
The authors report no conflicts or financial interests.
Acknowledgments
The authors acknowledge the financial contributions of the Saskatchewan Ministry of Agriculture, PrioNet Canada and the National Sciences and Engineering Research Council.
Supplemental Material
Supplemental material may be found here:
http://www.landesbioscience.com/journals/prion/article/21914/
References
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136 - 44; http://dx.doi.org/10.1126/science.6801762; PMID: 6801762
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 1992; 356:577 - 82; http://dx.doi.org/10.1038/356577a0; PMID: 1373228
- Roucou X, LeBlanc AC. Cellular prion protein neuroprotective function: implications in prion diseases. J Mol Med (Berl) 2005; 83:3 - 11; http://dx.doi.org/10.1007/s00109-004-0605-5; PMID: 15645198
- Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, et al. The cellular prion protein binds copper in vivo. Nature 1997; 390:684 - 7; http://dx.doi.org/10.1038/37733; PMID: 9414160
- Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet 2005; 6:435 - 50; http://dx.doi.org/10.1038/nrg1616; PMID: 15931169
- Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci U S A 2006; 103:2184 - 9; http://dx.doi.org/10.1073/pnas.0510577103; PMID: 16467153
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1:31 - 9; http://dx.doi.org/10.1038/35036052; PMID: 11413487
- Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta 2005; 1746:260 - 73; http://dx.doi.org/10.1016/j.bbamcr.2005.05.005; PMID: 15951036
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 2005; 308:1435 - 9; http://dx.doi.org/10.1126/science.1110837; PMID: 15933194
- Chesebro B, Race B, Meade-White K, Lacasse R, Race R, Klingeborn M, et al. Fatal transmissible amyloid encephalopathy: a new type of prion disease associated with lack of prion protein membrane anchoring. PLoS Pathog 2010; 6:e1000800; http://dx.doi.org/10.1371/journal.ppat.1000800; PMID: 20221436
- Mahal SP, Jablonski J, Suponitsky-Kroyter I, Oelschlegel AM, Herva ME, Oldstone M, et al. Propagation of RML prions in mice expressing PrP devoid of GPI anchor leads to formation of a novel, stable prion strain. PLoS Pathog 2012; 8:e1002746; http://dx.doi.org/10.1371/journal.ppat.1002746; PMID: 22685404
- Aguzzi A. Cell biology. Prion toxicity: all sail and no anchor. Science 2005; 308:1420 - 1; http://dx.doi.org/10.1126/science.1114168; PMID: 15933188
- Nixon RR. Prion-associated increases in Src-family kinases. J Biol Chem 2005; 280:2455 - 62; http://dx.doi.org/10.1074/jbc.M410883200; PMID: 15546860
- Marella M, Gaggioli C, Batoz M, Deckert M, Tartare-Deckert S, Chabry J. Pathological prion protein exposure switches on neuronal mitogen-activated protein kinase pathway resulting in microglia recruitment. J Biol Chem 2005; 280:1529 - 34; http://dx.doi.org/10.1074/jbc.M410966200; PMID: 15528202
- Lee HP, Jun YC, Choi JK, Kim JI, Carp RI, Kim YS. Activation of mitogen-activated protein kinases in hamster brains infected with 263K scrapie agent. J Neurochem 2005; 95:584 - 93; http://dx.doi.org/10.1111/j.1471-4159.2005.03429.x; PMID: 16135077
- Vassallo N, Herms J, Behrens C, Krebs B, Saeki K, Onodera T, et al. Activation of phosphatidylinositol 3-kinase by cellular prion protein and its role in cell survival. Biochem Biophys Res Commun 2005; 332:75 - 82; http://dx.doi.org/10.1016/j.bbrc.2005.04.099; PMID: 15896301
- Carimalo J, Cronier S, Petit G, Peyrin JM, Boukhtouche F, Arbez N, et al. Activation of the JNK-c-Jun pathway during the early phase of neuronal apoptosis induced by PrP106-126 and prion infection. Eur J Neurosci 2005; 21:2311 - 9; http://dx.doi.org/10.1111/j.1460-9568.2005.04080.x; PMID: 15932590
- Monnet C, Gavard J, Mège RM, Sobel A. Clustering of cellular prion protein induces ERK1/2 and stathmin phosphorylation in GT1-7 neuronal cells. FEBS Lett 2004; 576:114 - 8; http://dx.doi.org/10.1016/j.febslet.2004.08.076; PMID: 15474021
- Schneider B, Mutel V, Pietri M, Ermonval M, Mouillet-Richard S, Kellermann O. NADPH oxidase and extracellular regulated kinases 1/2 are targets of prion protein signaling in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A 2003; 100:13326 - 31; http://dx.doi.org/10.1073/pnas.2235648100; PMID: 14597699
- Tagliavini F, Prelli F, Verga L, Giaccone G, Sarma R, Gorevic P, et al. Synthetic peptides homologous to prion protein residues 106-147 form amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A 1993; 90:9678 - 82; http://dx.doi.org/10.1073/pnas.90.20.9678; PMID: 8105481
- Fabrizi C, Silei V, Menegazzi M, Salmona M, Bugiani O, Tagliavini F, et al. The stimulation of inducible nitric-oxide synthase by the prion protein fragment 106--126 in human microglia is tumor necrosis factor-α-dependent and involves p38 mitogen-activated protein kinase. J Biol Chem 2001; 276:25692 - 6; http://dx.doi.org/10.1074/jbc.M100133200; PMID: 11316802
- O’Donovan CN, Tobin D, Cotter TG. Prion protein fragment PrP-(106-126) induces apoptosis via mitochondrial disruption in human neuronal SH-SY5Y cells. J Biol Chem 2001; 276:43516 - 23; http://dx.doi.org/10.1074/jbc.M103894200; PMID: 11533027
- Pietri M, Caprini A, Mouillet-Richard S, Pradines E, Ermonval M, Grassi J, et al. Overstimulation of PrPC signaling pathways by prion peptide 106-126 causes oxidative injury of bioaminergic neuronal cells. J Biol Chem 2006; 281:28470 - 9; http://dx.doi.org/10.1074/jbc.M602774200; PMID: 16864581
- Thellung S, Villa V, Corsaro A, Arena S, Millo E, Damonte G, et al. p38 MAP kinase mediates the cell death induced by PrP106-126 in the SH-SY5Y neuroblastoma cells. Neurobiol Dis 2002; 9:69 - 81; http://dx.doi.org/10.1006/nbdi.2001.0461; PMID: 11848686
- Arsenault RJ, Jalal S, Babiuk LA, Potter A, Griebel PJ, Napper S. Kinome analysis of Toll-like receptor signaling in bovine monocytes. J Recept Signal Tr (2009), 299-311.
- Booth JS, Arsenault R, Napper S, Griebel PJ, Potter AA, Babiuk LA, et al. TLR9 signaling failure renders Peyer’s patch regulatory B cells unresponsive to stimulation with CpG oligodeoxynucleotides. J Innate Immun 2010; 2:483 - 94; http://dx.doi.org/10.1159/000316574; PMID: 20551621
- Storkebaum E, Carmeliet P. VEGF: a critical player in neurodegeneration. J Clin Invest 2004; 113:14 - 8; PMID: 14702101
- Weise J, Sandau R, Schwarting S, Crome O, Wrede A, Schulz-Schaeffer W, et al. Deletion of cellular prion protein results in reduced Akt activation, enhanced postischemic caspase-3 activation, and exacerbation of ischemic brain injury. Stroke 2006; 37:1296 - 300; http://dx.doi.org/10.1161/01.STR.0000217262.03192.d4; PMID: 16574930
- Paitel E, Fahraeus R, Checler F. Cellular prion protein sensitizes neurons to apoptotic stimuli through Mdm2-regulated and p53-dependent caspase 3-like activation. J Biol Chem 2003; 278:10061 - 6; http://dx.doi.org/10.1074/jbc.M211580200; PMID: 12529324
- Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res 2001; 61:1862 - 8; PMID: 11280739
- Kristensson K, Feuerstein B, Taraboulos A, Hyun WC, Prusiner SB, DeArmond SJ. Scrapie prions alter receptor-mediated calcium responses in cultured cells. Neurology 1993; 43:2335 - 41; http://dx.doi.org/10.1212/WNL.43.11.2335; PMID: 8232952
- Chow CW, Rincón M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 1997; 278:1638 - 41; http://dx.doi.org/10.1126/science.278.5343.1638; PMID: 9374467
- Loubet D, Dakowski C, Pietri M, Pradines E, Bernard S, Callebert J, et al. Neuritogenesis: the prion protein controls β1 integrin signaling activity. FASEB J 2012; 26:678 - 90; http://dx.doi.org/10.1096/fj.11-185579; PMID: 22038049
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001; 410:37 - 40; http://dx.doi.org/10.1038/35065000; PMID: 11242034
- Östlund P, Lindegren H, Pettersson C, Bedecs K. Altered insulin receptor processing and function in scrapie-infected neuroblastoma cell lines. Brain Res Mol Brain Res 2001; 97:161 - 70; http://dx.doi.org/10.1016/S0169-328X(01)00316-3; PMID: 11750072
- Rieger R, Edenhofer F, Lasmézas CI, Weiss S. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med 1997; 3:1383 - 8; http://dx.doi.org/10.1038/nm1297-1383; PMID: 9396609
- Jalal S, Arsenault R, Potter AA, Babiuk LA, Griebel PJ, Napper S. Genome to kinome: species-specific peptide arrays for kinome analysis. Sci Signal 2009; 2:pl1; http://dx.doi.org/10.1126/scisignal.254pl1; PMID: 19155530
- Li Y, Arsenault RJ, Trost B, Slind J, Griebel PJ, Napper S, et al. A systematic approach for analysis of peptide array kinome data. Sci Signal 2012; 5:pl2; http://dx.doi.org/10.1126/scisignal.2002429; PMID: 22510468
- Lynn DJ, Winsor GL, Chan C, Richard N, Laird MR, Barsky A, et al. InnateDB: facilitating systems-level analysis of the mammalian innate immune response. Mol Syst Biol 2008; 4:1 - 11; http://dx.doi.org/10.1038/msb.2008.55