Abstract
Prion diseases are a group of neurodegenerative disorders affecting humans as well as captive and wild animals. The mechanisms and routes governing the natural spread of prions are not completely understood and several hypotheses have been proposed. In this study, we analyzed the effect of gender in prion incubation period, as well as the possibility of prion transmission by sexual and parental contact using 263K infected hamsters as a model. Our results show that males have significantly longer incubation periods compared with females when exposed to the same quantity of infectious material. Importantly, no evidence of sexual or parental prion transmission was found, even 500 d after sexual contact or birth, respectively. Western blotting and PMCA were unable to detect sub-clinical levels of PrPSc in experimental subjects, suggesting a complete absence of prion transmission by these routes. Our results show that sexual and parental transmission of prions does not occur in this model. It remains to be studied whether this conclusion is valid also for other prion strains and species.
Introduction
Transmissible spongiform encephalopathies (TSEs) or prion diseases are a group of rare disorders affecting several mammalian species, including humans.Citation1,Citation2 These diseases could be inherited or acquired by infection, although the vast majority of cases in humans are sporadic.Citation3 The main histopathological features of these disorders include spongiform brain degeneration and the accumulation of an abnormally folded protein, termed PrPSc.Citation1 In humans, one every million people is estimated to be affected by these diseases each year.Citation4 This proportion is higher in some animal species, for example, Chronic wasting disease (CWD) which affect deer and elk is now epidemic in the United States and some Canadian provinces.Citation5 The high increase and spread of CWD cases have placed forward important concerns in terms of the mechanisms of transmission and the putative consequences it could have in the case of strain mutation when other species (including humans) are in contact with affected animals. A similar scenario, has been observed previously for sheep affected by scrapie.Citation6
Susceptibility to TSEs has been associated to several risk factors, such as polymorphisms in the host’s prion protein, age, gender, and environmental factors, among others.Citation7-Citation12 The influence of the gender on prion infectivity has been tested with somehow contradictory results.Citation12-Citation16 Whereas two studies showed no differences in the incubation periods between male and female mice after intra-cerebral (i.c.) injection of ME7 prion strain,Citation14,Citation16 another study showed longer incubation periods for males infected by i.c. and intra-peritoneal (i.p.) routes using the same agent.Citation15 While some strains have shown a gender dependency in their incubation periods, others have shown no effect.Citation16 Importantly, it has been established for vCJD that the age of onset is two years earlier in females than in males after stratification of the cohort by birth.Citation15
As previously mentioned, one of the most important questions yet to answer in the prion field involves the mechanisms of spread and transmission of the agent, especially in natural cases. It has been proposed that carcasses from prion infected animals as well as excreta (saliva, urine, feces, and placenta) carrying infectious prions could enter and progressively accumulate in the environment.Citation17 Prions bind tightly to soil and remain infectious after years in this material.Citation18-Citation21 Another mechanism proposed involves maternal transmission. Several lines of evidence have been provided for this route. For example, infectious material has been detected in placenta and mammary glands of infected dams.Citation22,Citation23 Moreover, the presence of the infectious material has also been identified by protein misfolding cyclic amplification (PMCA) in fetuses.Citation24 In addition, it has been reported that lambs from infected dams are in a significant risk to develop scrapie.Citation25 Although many studies have shown a positive correlation between infected mothers and the chance to develop scrapie, embryo transfer experiments suggested that if a mother-to-offspring transmission exists, it happens post-natal during lambing or suckling.Citation26 Many of the mother-to-offspring prion transmission studies done in sheep or rodents, involve the use of inter-species prion infection or PrP polymorphic variants that could mask the contribution of these routes on disease transmission.Citation25-Citation27 A potential route for prion transmission that remains poorly explored involves sexual contact. Although few studies have been done to explore the presence of infectious prions in semen from scrapie affected animals,Citation28,Citation29 no infectivity (at least to our knowledge) has been reported in testes or any other sexual tissue from male or female animals.
The purpose of this study is to investigate sexual and parental transmission of prions using a well-established animal model of prion diseases (Syrian hamster). Experimental subjects were infected with the well-characterized 263K prion strain that has been previously reported to have PrPSc widely disseminated in several peripheral tissues.Citation30,Citation31
Results
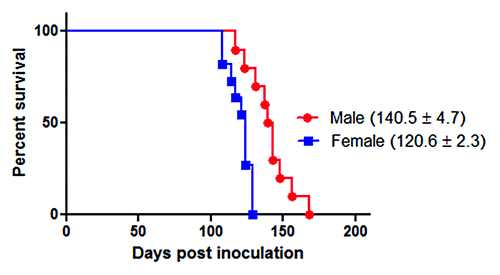
Male and female Golden Syrian hamsters (Mesocricetus auratus) were i.p. inoculated with the 263K prion strain (; ). The female group showed clinical signs and incubation periods consistent with previous results obtained in our lab. Interestingly, males showed significantly longer incubation periods (~14%) compared with females (). Both groups were injected at the same age and date with the same 263K source, minimizing the chances of variability. These results, which were in agreement with previous reports in some mouseCitation15,Citation16 and hamsterCitation12 prions strains as well as epidemiological data on vCJD,Citation15 suggest that males have a lower susceptibility to prion infectivity than females.
Figure 1. Breeding schemes and experimental groups. Three experimental groups were designed in order to investigate a possible sexual and parental transmission of prion disease. (A) Male and female hamsters were i.p. injected with 263K prions. Animals were sacrificed at stage 4 of the disease as previously reportedCitation46 and sexual organs were collected to assess PrPSc content by WB and PMCA. (B) Breeding pairs using different combinations of infected and un-infected males and females were set in order to assess a putative prion transmission by sexual contact. (C) Pups generated from breeding in (B) were kept and observed for appearance of prion disease.
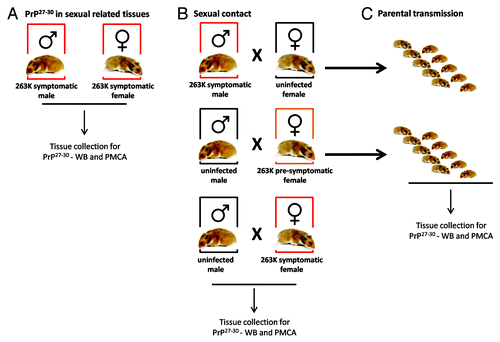
Table 1. Summary of groups, conditions and results obtained
Figure 2. Survival curves of intra-peritoneally infected 263K male and female Syrian hamsters. ~40 d old male (n = 10) and female (n = 11) hamsters were intra-peritoneally infected with 263K prions as described in Materials and Methods. Animals were sacrificed at advanced stage of clinical disease. Numbers in parenthesis note average incubation periods ± standard error. Survival curves were significantly different (P value = 0.0007).
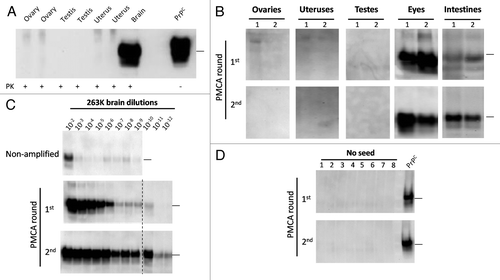
Sexual related organs including testes, uteruses and ovaries were collected from terminally sick male and female subjects and their PrPCitation27-Citation30 content was analyzed by western blot (WB) and PMCA. As shown in , the disease-associated form of the protein was absent in the sexual organs analyzed. To further study the presence of low concentrations of PrPSc in these tissues, we subjected the samples to PMCA. One or 2 rounds of PMCA cycles did not show any positive signal in the sexual organs (). As a control, eyes and intestines were also tested for their PrPSc content, showing positive results even in the first round of PMCA. Our positive control using serial dilutions of 263K infected hamsters brain, showed that after 2 rounds of PMCA we were able to detect the equivalent to a 10−12 brain dilution (), which is ~1000–10 000 times lower than the last dilution expected to cause disease by the i.c. route.Citation32 The level of amplification using the current, optimized PMCA setting is substantially more sensitive than the previous versions of the PMCA technology,Citation31,Citation33,Citation34 and permits much faster detection, minimizing the possibility for cross-contamination. Un-seeded PMCA controls, done in 8 replicates, did not show presence of contamination/de novo PrPSc generation ().
Figure 3. Western blotting and PMCA assessment of PrPSc in sexual organs. Male and female 263K infected hamsters were sacrificed at the clinical stage of the disease and sexual organs (ovaries, uteruses and testes) were collected. (A) WB analyses of PK treated tissue homogenates from selected animals. (B) PMCA analyses of same organs showed in (A) plus ocular and intestinal tissue homogenates. For space constrains, the results of 2 representative animals of the 10 studied are shown (1 and 2). (C) PMCA of brain dilutions from a sarkosyl cleared brain homogenate used as a positive control of in vitro amplification. (D) Un-seeded PMCA reaction used as a negative control. All PMCA generated samples (B–D) were PK treated before WB. PrPC correspond to brain homogenates from healthy hamsters (no PK treated) used as a control of electrophoretic mobility. Black horizontal lines at the right of each gel represent a 26 KDa molecular weight marker. Dotted line depicts splicing of two different gels. Numbers in (B) and (D) indicate samples from different animals.
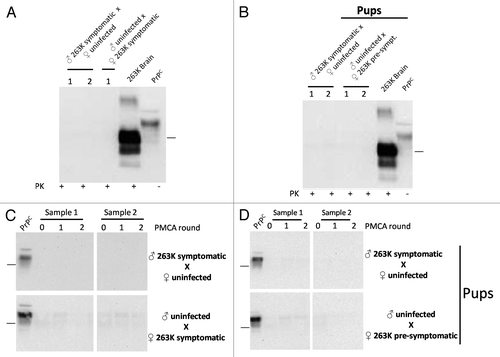
To investigate the putative transmission of prions through sexual contact, we arranged breeding groups using selected 263K infected and un-infected hamsters (). Two of the experimental groups described in this study involved the use of symptomatic males and females at 110 d post inoculation (dpi), which were bred with un-infected subjects. Another group involved breeding of un-infected males with infected females before showing clinical signs (70 dpi). The objectives of the first two groups were to (1) evaluate the possibility of transmission after sexual contact with individuals reaching the clinical stages of the disease, and (2) in the case of the groups involving the infected male and un-infected female, see the possibility of paternal transmission to new pups. For the experiment involving the pre-symptomatic mother, the purposes were to evaluate (1) whether an individual incubating the disease could transmit the illness to sexual partners, and (2) maternal transmission, allowing enough time for these mothers to carry and feed their pups before succumbing to prion pathology. As shown in , breeding was successful in 88.8% of early-symptomatic (males) or pre-symptomatic (females) animals (8/9; ). However, due to the specific signs of the 263K strain (including aggressiveness and hyper-sensitivity to tact, among others) only 1 out of 4 un-infected males were able to mate with symptomatic females. All un-infected sexual partners and pups were observed for clinical signs for ≥500 d after sexual contact or days of age, respectively. None of the un-infected sexual partners, nor any of the pups, showed any type of prion associated clinical signs during the course of the experiment (). After sacrificing, brain samples were collected from all experimental subjects and the presence of sub-clinical prion disease was evaluated by analyzing the content of PrPCitation27-Citation30 by WB and PMCA. As shown in , all results were negative. Importantly, PMCA efficiency in the setting used for this specific experiment was the same as the one depicted in , i.e., capable to detect up to a 10−12 dilution of 263K brain homogenate, which corresponds to the equivalent of a single particle of PrPSc.Citation34
Figure 4. Biochemical assessment of PrPSc after sexual and parental prion contact. Brain homogenates from males and females having sexual contact with infected animals were tested for WB (A) and PMCA (C). The brain of the uninfected animal depicted in the breeding is the one tested for PrPCitation27-Citation30 by either WB or PMCA. Brain homogenates of pups coming from infected mothers or fathers were also tested by WB (B) and PMCA (D). All PMCA generated samples (C, D) were PK treated before WB. The samples showed in panel d correspond to the brain of either male or females born from the breeding illustrated in the respective blot. 263K brain and PrPC (PK and non-PK treated, respectively) corresponds to brain homogenates from infected and healthy hamsters used as a control of electrophoretic mobility. Black horizontal lines at the right of each gel represent a 26 KDa molecular weight marker. Numbers 1 and 2 in (A) and (B) and samples 1 and 2 in panels (C) and (D) indicate samples coming from different animals, which are representative of all animals analyzed.
Discussion
One of the main concerns in the prion field is the elucidation of the mechanisms responsible for the spreading of natural prion diseases. Currently, several hypotheses have been proposed, including: horizontal transmission through direct contact,Citation17 environmental contamination,Citation17 spread by scavengers,Citation35,Citation36 and sexual and parental exposure.Citation25,Citation27,Citation29,Citation37 Related to the vertical routes, many reports involved inter-species and inter-polymorphic prion transmissions, which confuse the interpretation of the results due to the barriers and strain variation expected to appear as a consequence.Citation38
The main purpose of this work was to evaluate a possible sexual and parental transmission of prion disease using a cloned and widely characterized prion strain (263K) in the homologous species (Syrian hamster). Experimentally infected hamsters have been extensively used in prion research and are considered an excellent disease model. In addition, 263K prions have been proved to be widely disseminated in peripheral tissues,Citation30,Citation31 which accumulate the infectious agent even before animals show any clinical sign of the disease.Citation39 In order to increase the chances of peripheral dissemination, animals were i.p. injected. Infected subjects were analyzed for the appearance of prion disease, as well as their potential to transmit the disease by sexual contact or to their progeny.
Our results showed that males exhibit a significantly longer incubation period than females when both genders were infected with the same quantity and under the same conditions (). As previously mentioned, these results were consistent with those previously reported by Kimberlin and Walker,Citation12 as well as for other rodent prion models,Citation15,Citation16 and vCJD cases in humans.Citation15 One of these reports suggested that androgens might be responsible for the delayed disease onset in males.Citation15 It is important to mention that the PrP levels and other neuropathological changes were not different between males and females, regardless of the incubation periods.Citation16 Although our results showed a clear difference in the incubation periods between males and females, it is possible that the gender effect in prion disease could be strain dependent.
The next aim was to investigate whether the prion infectious agent was present in sexually-related organs. Our results showed no presence of the infectious agent by WB and PMCA in testes, ovaries and uteruses (). Our PMCA detection limit after 2 rounds of the optimized technology reached an equivalent to a 10–12 brain dilution, which according to mathematical estimations would contain between 20–50 units of PrP monomers, which would be in the expected range for a single particle of PrPSc oligomer.Citation34 Thus, the lack of detection by PMCA suggests that no molecules of the infectious agent were present in these tissues. Although, we cannot rule out the presence of PMCA inhibitors in the tissues analyzed that could make PMCA less efficient, we processed the samples with sarkosyl coupled to ultracentrifugation, a procedure that has been previously described to reduce the concentration of blood components and other molecules able to interfere with in vitro prion replication by PMCA.Citation32,Citation40 The known low levels of PrPC expression present in many peripheral tissues may provide a possible explanation for the lack of PrPSc presence in these organs. A previous study showed that although PrPC levels in the hamster ovaries have been described as undetectable by WB, testes exhibit a detectable signal, similar to the one found for intestines.Citation41 Interestingly, in our study we observed a clear PrPSc signal in intestines (), suggesting that the low level of PrPC expression does not completely explain the lack of prions in sexual organs. The results presented in this report show no indication of sexual transmission of 263K prions. Sub-clinical disease was also discarded by the negative results obtained after WB and PMCA assessment.
Another possible mechanism of prion spread between animals involves a parents-to-offspring transmission. In our experiment, pups coming from both, infected mothers or fathers did not generate clinical or sub-clinical prion disease (). In the case of pups from infected fathers, previous studies in sheep suggested that lambs coming from infected rams are not at increased risk of scrapie.Citation25 However, risk strongly increases when dams were clinically or sub-clinically affected by the disease.Citation25 Previous reports indicated that a possible maternal transmission of prions occurs after birth and not during gestation.Citation26,Citation42 In our experiment, infected mothers were bred before showing clinical signs in order to allow sufficient time for gestation and feeding the pups, before the terminal stage of the disease. All mothers included in this part of the experiment showed signs after delivery and fed pups at their clinical stage. Other experiments have shown the presence of protease resistant PrP and infectivity in the mammary glands and milk of sheep suffering from mastitis,Citation22,Citation43 event that could increase the chances of prion transmission to newborns. Inflammation processes in the mammary glands of female hamsters were not included in this study. Although the presence of PrPSc has been recently reported in sheep fetuses by PMCA,Citation24 it was not addressed whether the agent was present in quantities sufficient to cause disease. Our negative results are consistent with the lack of disease in people born from CJD-affected mothers.Citation44,Citation45
Although our in vitro analysis of the animals confirmed the in vivo results, the limited number of subjects used in our experiments does not permit to rule out a low level of transmission by sexual or parental routes. We also cannot rule out that distinct results may be obtained using a different host or prion strain, considering the widely known differences of tropisms and peripheral distribution of diverse prion strains.
Materials and Methods
Inoculum preparation and characterization
263K prions were obtained from the brain of a clinically sick (stage 4 of the disease, as described below) animal produced by i.p. prion infection. Frozen brain was homogenized at 10% (w/v) in phosphate buffer (PBS; HyClone. SH30256.01) containing a cocktail of protease inhibitors (Roche, 11697498001). Homogenate was spun down at 805 g for 45 s and resulting pellet was discarded. Presence of PrPCitation27-Citation30 was confirmed by WB as explained below. Samples were stored at –20 °C.
Hamster inoculation, breeding procedures, and weaning
Syrian Golden hamsters (Mesocricetus auratus) were obtained from Harlan®. An amount of 100 µL of 263K brain homogenate were i.p. injected into ~40 d old male (n = 10) and female (n = 11) hamsters. Animals were evaluated 5 d per week for appearance of prion clinical signs as previously described.Citation46 Briefly, clinical signs were assessed using the following scoring system: (1), normal animal; (2), mild behavioral abnormalities including hyperactivity and hypersensitivity to noise; (3), moderate behavioral problems including head tremors, ataxia, wobbling gait, head bobbing, irritability, and aggressiveness; (4), severe behavioral abnormalities including all of the above plus head and body jerks and spontaneous backrolls; and (5), terminal stage of the disease in which the animal lies in the cage and is no longer able to stand up. Animals staying at stage 4 for longer than 1 week were sacrificed by CO2 inhalation and tissues were collected for histopathological analyses. Incubation periods were defined from injection to sacrifice. Selected 263K infected male and female hamsters were bred with non-infected animals (~60 d old) in different groups (). (1) non-infected females × infected males at 110 d post-inoculation (dpi) (early stage of prion disease); (2) non-infected males × infected females at 70 dpi (pre-symptomatic stage, without clinical signs); and (3) non-infected males × infected females at 110 dpi (early/medium stage 2 of prion disease). Breeding was performed by placing together 1 male and 1 female in a clean cage for ~1h, repeating the process for 2 weeks. Females were receptive for breeding approximately every 4 d. They were identified by a thick secretion in the genital area and by breeding posture (lowering their chests and raising their tails) when in contact with males. When sexual contact was positive, it occurred repeatedly over the session. Animals were housed in groups of 5. Pregnant females were separated from cage mates as soon as identified and weaning of pups was performed 21 d after birth (separated by gender). Un-infected subjects and pups were observed for clinical signs ≥500 after contact or birth, respectively. Animals were sacrificed by CO2 inhalation and tissues were collected, stored at –80 °C, and used for WB and PMCA analyses. Some animals were sacrificed before the experimental endpoint due to health issues unrelated to prion infection. All animal procedures were in agreement with NIH guidelines and approved by the Animal Welfare Committee of the University of Texas Medical School at Houston.
Western blotting of PrPCitation27-Citation30
Western blotting of PrPCitation27-Citation30 was performed as previously described.Citation40 Briefly, 10% w/v brain homogenates were prepared as mentioned above and 19 µL of the sample were mixed with proteinase K (PK) (Sigma-Aldrich, P2308) at 50 µg/mL final concentration. Samples were digested for 1h at 37 °C in an Eppendorf® Thermomixer (450 rpm). PK reaction was stopped by adding 10 µL of LDS (4×) loading buffer (Invitrogen,) and samples were fractionated in NuPAGE gels (Invitrogen, NP0321BOX). Gels were transferred to nitrocellulose membranes (GE Healthcare, RPN303D) and probed with the 6D11 monoclonal antibody (Covance, SIG-39810). After incubation with secondary antibody (GE Healthcare, NA931V) and washing, PrPCitation27-Citation30 was visualized by chemoluminescence using ECL plus (GE Healthcare, RPN2132) in a dark chamber (BioRad®).
PMCA assay
A detailed explanation of the PMCA procedures can be reviewed in Morales et al.Citation40 For tissues from infected animals, samples were homogenized at 10% w/v and 1 mL was mixed with the same volume of a 20% sarkosyl solution (prepared in water) and concentrated by ultracentrifugation (146 000 g for 1 h at 4 °C) in a L8-70M Beckman-Coulter® ultracentrifuge. Supernatant was discarded and pellet was washed (without resuspension) with 2 mL of PBS. A new centrifugation procedure was performed at the same speed and temperature explained above for 30 min. Pellets were resuspended in 100 µL hamster PMCA substrate and 2 rounds of PMCA were performed. In order to dissociate pellets, the first PMCA round was performed for 72 h. Second round was performed for 24 h. An amount of 10 µL of brain homogenates from un-infected breeding partners and offspring was mixed with 90 µL of hamster PMCA substrate and submitted to 2 PMCA rounds (48 h each). Positive control consisted of a serially diluted “sarkosyl cleared” 263K brain homogenate.Citation31,Citation40 Unseeded PMCA reactions were used to control contamination/de novo generation of prions. Presence of PMCA amplified PrPSc was evaluated by WB after PK digestion as explained above.
Statistical analysis
Data were expressed as means ± standard error (SEM). Log-rank (Mantel-Cox) test was used to determine differences among the groups using the Graph Pad prism software, version 5.0. Statistical differences were considered significant for values of P < 0.05.
| Abbreviations: | ||
| TSEs | = | transmissible spongiform encephalopathies |
| PrPSc | = | disease-associated abnormal prion protein |
| PrPC | = | cellular prion protein |
| PrP27-30 | = | PrP core fragment resistant to protease degradation |
| CWD | = | chronic wasting disease |
| vCJD | = | variant Creutzfeldt-Jakob disease |
| BSE | = | bovine spongiform encephalopathy |
| PMCA | = | protein misfolding cyclic amplification |
| WB | = | Western blot |
Disclosure of Potential Conflict of Interest
Soto C is inventor on several patents related to the PMCA technology and is currently Founder, Chief Scientific Officer and Vice-President of Amprion Inc., a biotech company focusing on the commercial exploitation of PMCA for prion diagnosis. Morales R is listed as an inventor on one patent application related to the PMCA technology.
Acknowledgments
The authors would like to thank Dr Manuel Camacho for technical help with some experiments, Andrea Flores for animal care and María-José Liberona for critical review of the manuscript. This work was supported by NIH grants R01NS049173 and P01AI077774 to Soto C.
References
- Prusiner SB. Prions. Proc Natl Acad Sci U S A 1998; 95:13363 - 83; http://dx.doi.org/10.1073/pnas.95.23.13363; PMID: 9811807
- Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol 2008; 3:11 - 40; http://dx.doi.org/10.1146/annurev.pathmechdis.3.121806.154326; PMID: 18233951
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 2001; 24:519 - 50; http://dx.doi.org/10.1146/annurev.neuro.24.1.519; PMID: 11283320
- Harries-Jones R, Knight R, Will RG, Cousens S, Smith PG, Matthews WB. Creutzfeldt-Jakob disease in England and Wales, 1980-1984: a case-control study of potential risk factors. J Neurol Neurosurg Psychiatry 1988; 51:1113 - 9; http://dx.doi.org/10.1136/jnnp.51.9.1113; PMID: 3066847
- Williams ES. Chronic wasting disease. Vet Pathol 2005; 42:530 - 49; http://dx.doi.org/10.1354/vp.42-5-530; PMID: 16145200
- Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev Sci Tech 1996; 15:827 - 52; PMID: 9025137
- Baylis M, Goldmann W. The genetics of scrapie in sheep and goats. Curr Mol Med 2004; 4:385 - 96; http://dx.doi.org/10.2174/1566524043360672; PMID: 15354869
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB. Distinct prion proteins in short and long scrapie incubation period mice. Cell 1987; 51:651 - 62; http://dx.doi.org/10.1016/0092-8674(87)90134-6; PMID: 2890436
- Hunter N, Dann JC, Bennett AD, Somerville RA, McConnell I, Hope J. Are Sinc and the PrP gene congruent? Evidence from PrP gene analysis in Sinc congenic mice. J Gen Virol 1992; 73:2751 - 5; http://dx.doi.org/10.1099/0022-1317-73-10-2751; PMID: 1357080
- Dickinson AG, Meikle VM, Fraser H. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J Comp Pathol 1968; 78:293 - 9; http://dx.doi.org/10.1016/0021-9975(68)90005-4; PMID: 4970191
- Collinge J, Palmer MS, Dryden AJ. Genetic predisposition to iatrogenic Creutzfeldt-Jakob disease. Lancet 1991; 337:1441 - 2; http://dx.doi.org/10.1016/0140-6736(91)93128-V; PMID: 1675319
- Kimberlin RH, Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol 1977; 34:295 - 304; http://dx.doi.org/10.1099/0022-1317-34-2-295; PMID: 402439
- Outram GW. The pathogenesis of scrapie in mice. Front Biol 1976; 44:325 - 57; PMID: 821791
- Abiola OO, Iyegbe C, Lantos P, Plomin R, Anderton BH, Whatley SA. Profound sex-specific effects on incubation times for transmission of bovine spongiform encephalopathy to mice. Intervirology 2002; 45:56 - 8; http://dx.doi.org/10.1159/000050088; PMID: 11937772
- Loeuillet C, Boelle PY, Lemaire-Vieille C, Baldazza M, Naquet P, Chambon P, Cesbron-Delauw MF, Valleron AJ, Gagnon J, Cesbron JY. Sex effect in mouse and human prion disease. J Infect Dis 2010; 202:648 - 54; http://dx.doi.org/10.1086/654818; PMID: 20594106
- Akhtar S, Wenborn A, Brandner S, Collinge J, Lloyd SE. Sex effects in mouse prion disease incubation time. PLoS One 2011; 6:e28741; http://dx.doi.org/10.1371/journal.pone.0028741; PMID: 22174884
- Bartelt-Hunt SL, Bartz JC. Behavior of prions in the environment: implications for prion biology. PLoS Pathog 2013; 9:e1003113; http://dx.doi.org/10.1371/journal.ppat.1003113; PMID: 23408883
- Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog 2006; 2:e32; http://dx.doi.org/10.1371/journal.ppat.0020032; PMID: 16617377
- Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. Scrapie Agent (Strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2007; 2:e435; http://dx.doi.org/10.1371/journal.pone.0000435; PMID: 17502917
- Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 2007; 3:e93; http://dx.doi.org/10.1371/journal.ppat.0030093; PMID: 17616973
- Brown P, Gajdusek DC. Survival of scrapie virus after 3 years’ interment. Lancet 1991; 337:269 - 70; http://dx.doi.org/10.1016/0140-6736(91)90873-N; PMID: 1671114
- Ligios C, Sigurdson CJ, Santucciu C, Carcassola G, Manco G, Basagni M, Maestrale C, Cancedda MG, Madau L, Aguzzi A. PrPSc in mammary glands of sheep affected by scrapie and mastitis. Nat Med 2005; 11:1137 - 8; http://dx.doi.org/10.1038/nm1105-1137; PMID: 16270061
- Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis 1998; 178:949 - 53; http://dx.doi.org/10.1086/515669; PMID: 9806020
- Garza MC, Fernández-Borges N, Bolea R, Badiola JJ, Castilla J, Monleón E. Detection of PrPres in genetically susceptible fetuses from sheep with natural scrapie. PLoS One 2011; 6:e27525; http://dx.doi.org/10.1371/journal.pone.0027525; PMID: 22194786
- Hoinville LJ, Tongue SC, Wilesmith JW. Evidence for maternal transmission of scrapie in naturally affected flocks. Prev Vet Med 2010; 93:121 - 8; http://dx.doi.org/10.1016/j.prevetmed.2009.10.013; PMID: 19945758
- Andréoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, Lantier F, Berthon P, Eychenne F, Lafond-Benestad S, Elsen JM, et al. PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol 2002; 83:2607 - 16; PMID: 12237445
- Foster JD, Goldmann W, McKenzie C, Smith A, Parnham DW, Hunter N. Maternal transmission studies of BSE in sheep. J Gen Virol 2004; 85:3159 - 63; http://dx.doi.org/10.1099/vir.0.80099-0; PMID: 15448379
- Sarradin P, Melo S, Barc C, Lecomte C, Andréoletti O, Lantier F, Dacheux JL, Gatti JL. Semen from scrapie-infected rams does not transmit prion infection to transgenic mice. Reproduction 2008; 135:415 - 8; http://dx.doi.org/10.1530/REP-07-0388; PMID: 18299435
- Rubenstein R, Bulgin MS, Chang B, Sorensen-Melson S, Petersen RB, LaFauci G. PrP(Sc) detection and infectivity in semen from scrapie-infected sheep. J Gen Virol 2012; 93:1375 - 83; http://dx.doi.org/10.1099/vir.0.038802-0; PMID: 22323531
- Kimberlin RH, Walker CA. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol 1986; 67:255 - 63; http://dx.doi.org/10.1099/0022-1317-67-2-255; PMID: 3080549
- Chen B, Morales R, Barria MA, Soto C. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat Methods 2010; 7:519 - 20; http://dx.doi.org/10.1038/nmeth.1465; PMID: 20512142
- Morales R, Buytaert-Hoefen KA, Gonzalez-Romero D, Castilla J, Hansen ET, Hlavinka D, Goodrich RP, Soto C. Reduction of prion infectivity in packed red blood cells. Biochem Biophys Res Commun 2008; 377:373 - 8; http://dx.doi.org/10.1016/j.bbrc.2008.09.141; PMID: 18851948
- Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med 2005; 11:982 - 5; PMID: 16127436
- Saá P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J Biol Chem 2006; 281:35245 - 52; http://dx.doi.org/10.1074/jbc.M603964200; PMID: 16982620
- Hamir AN, Miller JM, Cutlip RC, Stack MJ, Chaplin MJ, Jenny AL, Williams ES. Experimental inoculation of scrapie and chronic wasting disease agents in raccoons (Procyon lotor). Vet Rec 2003; 153:121 - 3; http://dx.doi.org/10.1136/vr.153.4.121; PMID: 12918830
- VerCauteren KC, Pilon JL, Nash PB, Phillips GE, Fischer JW. Prion remains infectious after passage through digestive system of American crows (Corvus brachyrhynchos). PLoS One 2012; 7:e45774; http://dx.doi.org/10.1371/journal.pone.0045774; PMID: 23082115
- Bencsik A, Debeer S, Petit T, Baron T. Possible case of maternal transmission of feline spongiform encephalopathy in a captive cheetah. PLoS One 2009; 4:e6929; http://dx.doi.org/10.1371/journal.pone.0006929; PMID: 19738899
- Morales R, Abid K, Soto C. The prion strain phenomenon: molecular basis and unprecedented features. Biochim Biophys Acta 2007; 1772:681 - 91; http://dx.doi.org/10.1016/j.bbadis.2006.12.006; PMID: 17254754
- Saá P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science 2006; 313:92 - 4; http://dx.doi.org/10.1126/science.1129051; PMID: 16825570
- Morales R, Duran-Aniotz C, Diaz-Espinoza R, Camacho MV, Soto C. Protein misfolding cyclic amplification of infectious prions. Nat Protoc 2012; 7:1397 - 409; http://dx.doi.org/10.1038/nprot.2012.067; PMID: 22743831
- Bendheim PE, Brown HR, Rudelli RD, Scala LJ, Goller NL, Wen GY, Kascsak RJ, Cashman NR, Bolton DC. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology 1992; 42:149 - 56; http://dx.doi.org/10.1212/WNL.42.1.149; PMID: 1346470
- Foster J, McKelvey W, Fraser H, Chong A, Ross A, Parnham D, Goldmann W, Hunter N. Experimentally induced bovine spongiform encephalopathy did not transmit via goat embryos. J Gen Virol 1999; 80:517 - 24; PMID: 10073715
- Ligios C, Cancedda MG, Carta A, Santucciu C, Maestrale C, Demontis F, Saba M, Patta C, DeMartini JC, Aguzzi A, et al. Sheep with scrapie and mastitis transmit infectious prions through the milk. J Virol 2011; 85:1136 - 9; http://dx.doi.org/10.1128/JVI.02022-10; PMID: 21084475
- Xiao X, Miravalle L, Yuan J, McGeehan J, Dong Z, Wyza R, MacLennan GT, Golichowski AM, Kneale G, King N, et al. Failure to detect the presence of prions in the uterine and gestational tissues from a Gravida with Creutzfeldt-Jakob disease. Am J Pathol 2009; 174:1602 - 8; http://dx.doi.org/10.2353/ajpath.2009.081045; PMID: 19349373
- Berrebi A, Cohen M, Ayoubi JM. [Creutzfeldt-Jakob disease and pregnancy]. J Gynecol Obstet Biol Reprod (Paris) 1997; 26:755 - 9; PMID: 9509315
- Castilla J, Gonzalez-Romero D, Saá P, Morales R, De Castro J, Soto C. Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell 2008; 134:757 - 68; http://dx.doi.org/10.1016/j.cell.2008.07.030; PMID: 18775309