Abstract
The shotgun strategy applying tandem mass spectrometry has been widely used to identify the proteins that are differentially distributed among diseases for its high reliability and efficiency. To find out the potential difference of protein profiles in cerebrospinal fluids (CSF) between Creutzfeldt-Jakob disease (CJD) and non-CJD patients, especially in the fraction ranging from 1–10 KD, the CSF samples of 40 probable sporadic CJD (sCJD) patients, 32 non-CJD cases with dementia and 17 non-CJD cases without dementia were separately pooled and enriched by the magnetic beads based weak cation exchange chromatography (MB-WCX). After trypsin digestion, each enriched CSF was separated and identified by RP-HPLC-ESI-QTOF MS/MS. In total, 42, 53 and 47 signals of proteins were identified in the pooled CSF fraction less than 10 KD of probable sCJD, non-CJD with dementia and non-CJD without dementia, respectively. Compared with that of probable sCJD, the similarity of CSF protein profiles of non-CJD with dementia (76.2%) were higher than that of non-CJD without dementia (57.1%). Nine CSF proteins were found to be specially observed in probable sCJD group. Those data may help to select the potential biomarkers for diagnosis of CJD. Additionally, further studies of the small segments of cellular proteins in CSF of CJD patients may also provide scientific clues for understanding the neuropathogenesis of TSEs.
Introduction
Creutzfeldt-Jakob disease (CJD) is characterized by rapidly progressive and ultimately fatal disorder of the central nervous system (CNS). The major clinical symptoms of CJD include rapidly progressive dementia, myoclonus, visual or cerebellar symptoms, pyramidal or extrapyramidal signs and akinetic mutism.Citation1 The definite diagnosis of CJD depends on the neuropathological examination and demonstration of the pathological isoform of the prion protein (PrPSc) in CNS, either at biopsy or autopsy.Citation2 Probable or possible diagnosis of CJD may achieve based on the combinations of the clinical manifestations, typical changes in electroencephalography (EEG) and the appearance or alternation of protein in cerebrospinal fluid (CSF).Citation3 Although several surrogate markers have showed diagnostic potential,Citation4 only immunoblot for CSF 14-3-3 is included in the diagnostic criteria up to now.Citation5,Citation6 Screenings of valid and reliable biomarkers and developments of diagnostic tools for CJD based on accessible specimen are on demand.
CSF plays an essential physiological role in homeostasis of neuronal cells. Changes in CSF composition may reflect physiological or pathological status of CNS. Actually, determinations of biomarkers in CSF have been already widely used in the diagnoses of certain CNS diseases. However, most of the targets of diagnostic methods focus on full-length proteins, whose relative molecular weights are usually larger than 10 kilodaltons (KD). The profiles of small proteins or degraded peptides from “biomarker” proteins in CSF of CNS diseases, especially CJD, still remain unknown.
The shotgun strategy applying tandem mass spectrometry based on proteomics has been widely used in identification of differential expression of proteins in the tissues and clinical samples of certain diseases, which are helpful not only for understanding the pathogenesis of disease, but also for screening useful biomarkers and developing diagnostic and prognostic methodologies. A magnetic beads based weak cation exchange chromatography (The Profiling Kit MB-WCX) has been developed for enrichment of small molecular weight proteins or peptides, ranging from 1–10 KD. Successful applications and reliable reproducibilities of MB-WCX beads in enrichments of small proteins in serum, plasma and urine have been described in some studies in references Citation7–Citation9.
To screen the profiles of proteins ranging from 1–10 KD in CSF of human TSE, panels of CSF from sporadic CJD (sCJD) and non-CJD patients were pooled. The expected portion of the peptides in CSF was enriched with Profiling Kit MB-WCX and analyzed with RP-HPLC-QTOF MS/MS. We found nine proteins specially presented in CSF of CJD. The identification and characterization of those proteins in CSF will provide the clue for searching biomarkers of CJD.
Results
Totally 40 CSF samples of probable sCJD, 32 of non-CJD with dementia and 17 of non-CJD without dementia were enrolled in this study. The CSF samples each group were pooled and processed with MB-WCX profiling kit for enrichment of the fraction of small peptides (<10 KD) as described above. To address the results after enrichment with the profiling kit MB-WCX, samples were subjected into MALDI-TOF. The results showed that the signal peaks of the peptides highly predominately concentrated within the mass range below 10 KD (). It implies that the enriched CSF samples with the profiling kit MB-WCX in present study correctly collect the peptide fractions below the mass range of 10 KD.
To look for the potential difference in the small peptides ranging 1–10 KD among the groups of probable sCJD and non-CJD, the enriched pooled CSF samples were separately analyzed with RP-HPLC-QTOF MS/MS after trypsin digestion. Subsequently, the data of MS/MS each group were searched in the NCBI database with the help of mascot search engine. Finally, the amino acid sequences of the identified proteins were comparatively analyzed, in order to find the same protein with different names and accession numbers. In total, 42, 52 and 47 proteins were identified in the fractions of mass range from 1–10 KD of the pooled CSF samples from probable sCJD, non-CJD with dementia and non-CJD without dementia, respectively (). The exact names of proteins in each group were summarized in Tables S1–3.
To address the similarity of the proteins in the fraction of mass range from 1–10 KD among various groups, the proteins that appeared both in the CSF samples of probable sCJD and in that of non-CJD were figure out. Thirty two out of forty two (76.2%) proteins in CSF of probable sCJD were also identified in that of non-CJD with dementia, whereas 24 (57.1%) in that of non-CJD without dementia (). The similarity of protein distributions in the CSF fraction less than 10 KD between probable sCJD and non-CJD with dementia was higher than that compared with non-CJD without dementia, but without significant statistical difference.
Comparison of the CSF proteins of the group of probable sCJD with that of two non-CJD groups revealed nine items that specially appeared in the fraction of mass range from 1–10 KD of the pooled CSF samples from probable sCJD. Those nine proteins were shown in . No PrP-like peptide was found in all tested CSF samples, whereas the segments of 14-3-3 protein, glial fibrillary acidic protein (GFAP) and apolipoprotein E (ApoE) were concurrently observed in the CSF samples from both sCJD and non-CJD.
Discussion
Human and animal TSEs have drawn great concern worldwide since the outbreak of bovine spongiform encephalopathy (BSE) and human variant CJD (vCJD) in the end of last century. Meanwhile it attracts lots of interests of scientists for its mysterious pathogen. Since TSEs usually do not have specific clinical symptoms and the pathogen is not detectable in the peripheral specimens, the definite diagnoses of TSEs still rely on the brain samples.Citation5 As a buffer bank in homeostasis of brain tissues, the alternations of the proteins in CSF during the pathogenesis of TSE have been repeatedly addressed, among them 14-3-3, tau, NSE and S100, are most commonly mentioned.Citation10,Citation11 Except 14-3-3, the diagnostic values of other markers for CJD still remain evaluations.
Most of protein-identified approaches up to now were focused on or at least restricted at the context of full-length protein, which are usually larger than 10 KD. In this study, we first screen the profiles of CSF protein in the fraction of small molecular mass (less than 10 KD) among probable sCJD and non-CJD patients, using a shotgun strategy applying tandem mass spectrometry based on peptidome. Nine proteins seem to be specially distributed in probable sCJD at our experimental condition. Among them, Cystatin CCitation12 and α-1-antichymotrypsin,Citation13 have been already reported to be possibly related to prion disease. Proteolipid protein has been described to be related to other neurological disorder, that is Pelizaeus-Merzbacher disease.Citation14 The potential roles of the rest in neurodegenerative diseases still remain unknown. Whether those biomarkers have possible meanings in the diagnosis or the pathogenesis of CJD are worth for further study.
The signals of proteins identified in our study here mostly restrict at the mass range below 10 KD, which represent the presences of the degraded form(s) of the proteins in CSF. Although we could not definitely exclude the possibility that the signals of small segments observed in the experiment derive from the respective full-length proteins after trypsin digestion, the MALDI-TOF data of the enriched fraction of the pooled CSF samples here illustrate a low likelihood. Being multiple lengths of cellular proteins in body fluids is a common phenomenon, which represents a regular metabolism process of protein or a degradation situation of protein during cell lysis.Citation15 Interestingly, the signals of several neurodegeneration-associated proteins, such as glial fibrillary acidic protein (GFAP) and apolipoprotein E (ApoE), even 14-3-3 protein, have been also identified in this fraction, but not limiting to the CSF samples of probable sCJD, which may indicate a common phenomenon of metabolism of cellular proteins in neurodegeneration.
The profiles of the proteins in the fraction less than 10 KD in the CSF samples of non-CJD with dementia show closer similarity to that of probable sCJD, compared with that of non-CJD without dementia. It seems that appearances of dementia may slightly influence the profiles of the small segments of proteins in CSF. The median age of probable sCJD is slightly older than that of two non-CJD groups, but without statistic difference. In line with the previous observation, methionin homozygous in codon 129 of PRNP are predominant in all groups. Therefore, ages and polymorphisms of codon 129 of the enrolled patients may have little effectiveness on the profiles of small molecular protein in CSF. Although there are a few patients showing CSF 14-3-3 positive in the groups of non-CJD, more cases in non-CJD without dementia (3/17, 17.6%) than that of non-CJD with dementia (1/32, 3.2%) may suggest that the presences of full-length 14-3-3 protein in CSF do not significantly affect the similarity of the profiles of small molecular proteins among the groups.
With new high-end proteomics technologies, we first provide the profiles of CSF proteins in the fraction less than 10 KD in the patients with probable sCJD, which are usually unable to be identified by routine techniques. Those data may help to select potential biomarkers for diagnosis of CJD. However, we have to admit that the present study has also obvious limitations. One is that the difference in profiles of CSF proteins between sCJD and non-CJD is merely based on qualitative screenings, which does not collect the information of the possible up or downregulating proteins quantitatively among groups. The other is that the sCJD patients enrolled in this study are probable sCJD and all patients in non-CJD are lacking of autopsy, hence, the possibility of misdiagnosis in each group is hard to definitely exclude. Notwithstanding, our study may provide useful scientific clues for further discovery. Systematical analyses of the small segments of cellular proteins in CSF of CJD patients will help us to understand the pathogenesis of TSE.
Materials and Methods
Ethics statement.
Usage of the stored human CSF samples in China CJD Surveillance System has been approved by the Research Ethics Committee of National Institute for Viral Disease Control and Prevention, China CDC.
Clinical samples.
In total, 40 CSF samples of probable sCJD and 49 samples of non-CJD, who did not fulfill the criteria for CJD, were included in this study. The diagnoses of CJD were made by China CJD Surveillance Centre according to WHO CJD diagnostic criteria as described previously in reference Citation5. All CSF samples were free of blood contamination. After being centrifuged at 3,000 rpm for 20 min, CSF samples were aliquotted and stored at −80°C until use. Total protein concentrations of CSF samples were determined by spectrophotometer. The concentrations of all samples were distributed in the normal range (CSF-Pro, 150–450 mg/l).
The CSF specimens were grouped as probable sCJD, and non-CJD. The group of non-CJD was further divided into two subgroups, based on whether having dementia or not during the clinical course. The main clinical features of each group were summarized in . The median ages at onset of the patients of probable sCJD and the ones of non-CJD with and without dementia, were 62 (range: 21–76), 51 (range: 15–77) and 54-y-old (range: 15–70), respectively. No age and gender differences were observed among three groups. Typical periodic sharp-wave complexes in EEG were observed in 35 out of 40 (87.5%) probable sCJD patients, 1 out of 32 non-CJD with dementia (3.1%) and 1 out of 17 non-CJD without dementia (5.9%), showing significantly difference (p < 0.01). CSF 14-3-3 proteins were positively detected in 67.5% probable sCJD cases, 3.2% non-CJD with dementia and 17.6% non-CJD without dementia, with significant difference (p < 0.01). Except two patients showing Met/Val in PRNP codon 129 the group of non-CJD with dementia, all cases were Met/Met in codon 129. Almost all probable sCJD patients displayed obviously progressive dementia in their clinical courses. Except for progressive dementia, the presences of other clinical manifestations, including myoclonus, visual or cerebella disturbance, pyramidal or extrapyramidal dysfunction, akinetic mutism and mental syndromes, in each group were also comparatively summarized in .
Peptidome enrichment.
Thirty microliters of CSF specimen each patient was taken and pooled based on the groups. For enrichment of peptides in CSF, 120 µl pooled CSF sample each group was subjected into a commercial MB-WCX kit (Bruker Daltonics, Bremen, Germany). Because of the volume limitation of the kit, the enrichment of 120 µl pooled CSF sample was simultaneously performed with four preparations according to the manufacturer's instruction. Briefly, 30 µl pooled CSF sample was diluted in 30 µl MB-WCX binding solution and mixed with 5 µl WCX beads (particle size <1 µm; mean pore size, 40 nm; specific surface area, 100 cm2/g). After thoroughly stirring, the mixtures were incubated at room temperature for 5 min. The beads in each tube were precipitated with the help of a magnetic separator and the supernatants were carefully removed by a pipette. After washing beads with 50 µl washing buffer, 2.5 µl of eluting buffer was added and dissolve the beads. The clear supernatant was collected and transferred into a fresh tube. Another 2.5 µl of eluting buffer was added the beads, making the final eluting volume of 5 µl. Four times of the eluting products in each group were mixed together, making the final volume of 20 µl that represented 120 µl pooled CSF samples.
Trypsin digestion.
The lyophilized trypsin (20 µg, 883 pM/l) was reconstituted in 25 mM/l ammonium bicarbonate solution (pH8.0) as working solution, with the final concentration of 20 µg/ml. An 5 µl of trypsin working solution was added to the each eluted pooled group and incubated at 37°C for 12 h. Subsequently, the digested samples were dried in a vacuum centrifuge.
Peptidome separation and data acquisition by nano RP-HPLC-ESI-QTOF.
The trypsin digested peptides were dissolved in 15 µl protein dissolution solution containing 50% acetonitrile (ACN) and 0.5% trifluoroacetic acid (Sigma-Aldrich), and separated and analyzed on Ultimate 3000 nanoHPLC (DIONEX) coupled to micrOTOFQ (Bruker Daltonics). A 10 µl solution of each sample was injected into the trap column with the autosampler of Ultimate 3000. The trap column was washed with 0.1% formic acid at a flow rate of 20 µl/min for 5 min in order to desalt the samples. The desalted peptides were then separated on a C18 column (packed in-house; Synergi C18; 150 × 0.075 mm) and analyzed on micrOTOF-Q mass spectrometer with a nanoelectrospray ionization ion source (ESI). The flow rate was maintained at 400 nl/min. The gradient was started at 3% ACN with 0.1% formic acid and linearly increased to 43% ACN in 40 min, then to 73% ACN in 5 min, and to 95% ACN in another 5 min. The gradient was then decreased to 3% ACN in 1 min and maintained at 3% ACN for 14 min. The mass spectrometer was operated in the positive ion MS mode, and data-dependent analysis was employed for survey scans (m/z 350–1,500) to choose up to three most intense precursor ions. For collision induced dissociation (CID) mass spectrometric (MS/MS) analysis, collision energies were chosen automatically as a function of m/z and charge. The collision gas was argon. The temperature of the heated sample source was 180°C, and the electrospray voltage was 1,400 V. External mass calibration in quadratic regression mode using sodium formiate resulted in mass errors of typically 5 ppm in the m/z range 50–2,000. Dynamic exclusion was continued for 1 min.
Bioinformatics.
Mass spectra were processed with DataAnalysis 4.0 (Bruker Daltonics) and the resulting MGF documents were searched for tryptic peptides with up to one miscleavage against NCBI human database using Mascot software (Matrix Science Ltd.). Both the peptide and MS/MS mass tolerance was set as ±0.1 Da. After the first round of mascot search, the second round of error tolerant search was performed against positive hits in the previous search. A criterion for significance was defined using a probability based Mascot score. Individual ions scores >54 indicate identity or extensive homology (p < 0.05).
Statistic assays.
All statistical analyses were performed using the SPSS 16.0 software.
Figures and Tables
Figure 1 The mass spectrums of the pooled CSF sample of probable sCJD patients (A), non-CJD cases with dementia (B) or without dementia (C). The mass spectrogram of MALDI-TOF illustrates most of signal peaks locate at the m/z range less than 10 KD after enrichment with profiling kit MB-WCX. Inserted figure shows the signal peaks in the m/z range from 1–15 KD. X-axis represents mass charge ratio and Y-axis represents intensity.
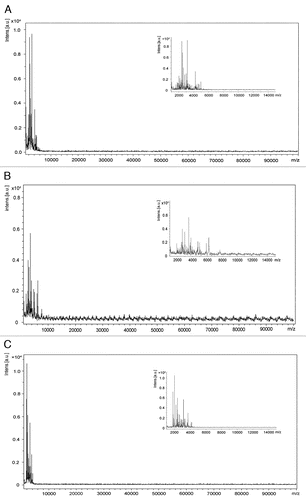
Table 1 Clinical characteristics of patient groups
Table 2 The protein number of Mascot search results and comparison of the similarity and specificity between probable CJD and non-CJD groups
Table 3 Screening result of proteins uniquely distributed in probable CJD compare to non-CJD group
Additional material
Download Zip (132.8 KB)Acknowledgments
This work was supported by Chinese National Natural Science Foundation Grants 30800975, National Basic Research Program of China (973 Program) (2007CB310505), China Mega-Project for Infectious Disease (2009ZX10004-101 and 2008ZX10004-008) and the SKLID Development Grant (2008SKLID102, 2011SKLID204 and 2011SKLID211).
References
- Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro Cuesta J, Knight RS, et al. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology 2000; 55:811 - 815; PMID: 10994001
- Budka H, Aguzzi A, Brown P, Brucher JM, Bugiani O, Gullotta F, et al. Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol 1995; 5:459 - 466; PMID: 8974629; http://dx.doi.org/10.1111/j.1750-3639.1995.tb00625.x
- Will RG. Epidemiology of Creutzfeldt-Jakob disease. Br Med Bull 1993; 49:960 - 970; PMID: 8137137
- Weber T, Otto M, Bodemer M, Zerr I. Diagnosis of Creutzfeldt-Jakob disease and related human spongiform encephalopathies. Biomed Pharmacother 1997; 51:381 - 387; PMID: 9452787; http://dx.doi.org/10.1016/S0753-3322(97)89430-9
- WHO manual for surveillance of human transmissible spongiform encephalopathies including variant Creutzfeldt-Jakob disease. WHO Communicable Disease Surveillance and Response 2003; Geneva
- Zerr I, Schulz-Schaeffer WJ, Giese A, Bodemer M, Schröter A, Henkel K, et al. Current clinical diagnosis in Creutzfeldt-Jakob disease: identification of uncommon variants. Ann Neurol 2000; 48:323 - 329; PMID: 10976638; http://dx.doi.org/10.1002/1531-8249(200009)48:3<323::AID-ANA6>3.0.CO;2-5
- Fiedler GM, Baumann S, Leichtle A, Oltmann A, Kase J, Thiery J, et al. Standardized peptidome profiling of human urine by magnetic bead separation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chem 2007; 53:421 - 428; PMID: 17272489; http://dx.doi.org/10.1373/clinchem.2006.077834
- Shin S, Cazares L, Schneider H, Mitchell S, Laronga C, Semmes OJ, et al. Serum biomarkers to differentiate benign and malignant mammographic lesions. J Am Coll Surg 2007; 204:1065 - 1071; PMID: 17481542; http://dx.doi.org/10.1016/j.jamcollsurg.2007.01.036
- Xiao D, Meng FL, He LH, Gu YX, Zhang JZ. Analysis of the urinary peptidome associated with Helicobacter pylori infection. World J Gastroenterol 2011; 17:618 - 624; PMID: 21350710; http://dx.doi.org/10.3748/wjg.v17.i5.618
- Green AJ, Thompson EJ, Stewart GE, Zeidler M, McKenzie JM, MacLeod MA, et al. Use of 14-3-3 and other brain-specific proteins in CSF in the diagnosis of variant Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 2001; 70:744 - 748; PMID: 11385008; http://dx.doi.org/10.1136/jnnp.70.6.744
- Chen C, Shi Q, Zhang BY, Wang GR, Zhou W, Gao C, et al. The prepared tau exon-specific antibodies revealed distinct profiles of tau in CSF of the patients with Creutzfeldt-Jakob disease. PLoS One 2010; 5:11886; PMID: 20686702; http://dx.doi.org/10.1371/journal.pone.0011886
- Sanchez JC, Guillaume E, Lescuyer P, Allard L, Carrette O, Scherl A, et al. Cystatin C as a potential cerebrospinal fluid marker for the diagnosis of Creutzfeldt-Jakob disease. Proteomics 2004; 4:2229 - 2233; PMID: 15274116; http://dx.doi.org/10.1002/pmic.200300799
- Miele G, Seeger H, Marino D, Eberhard R, Heikenwalder M, Stoeck K, et al. Urinary alpha1-antichymotrypsin: a biomarker of prion infection. PLoS One 2008; 3:3870; PMID: 19057641; http://dx.doi.org/10.1371/journal.pone.0003870
- Woodward K, Malcolm S. Proteolipid protein gene: Pelizaeus-Merzbacher disease in humans and neurodegeneration in mice. Trends Genet 1999; 15:125 - 128; PMID: 10203813; http://dx.doi.org/10.1016/S0168-9525(99)01716-3
- Roche S, Gabelle A, Lehmann S. Clinical proteomics of the cerebrospinal fluid: Towards the discovery of new biomarkers. Proteomics Clin Appl 2008; 2:428 - 436; http://dx.doi.org/10.1002/prca.200780040