Abstract
Loss of the Arabidopsis NFX1-LIKE2 (NFXL2) gene (At5g05660) results in elevated ABA levels, elevated hydrogen peroxide levels, reduced stomatal aperture, and enhanced drought stress tolerance. Introduction of the NFXL2–78 isoform into the nfxl2–1 mutant is largely sufficient for complementation of the phenotype. We show here that cuticular properties are altered in the nfxl2–1 mutant. The NFXL2–78 protein binds to the SHINE1 (SHN1), SHN2, SHN3, and BODYGUARD1 (BDG1) promoters and mediates weaker expression of these genes. The SHN AP2 domain transcription factors influence cuticle properties. Stronger SHN1, SHN2, and SHN3 expression in the nfxl2–1 mutant may cause altered cuticle properties including reduced stomatal density, and partly explain the enhanced drought stress tolerance. The BDG1 protein also controls cuticle development and is essential for osmotic stress regulation of ABA biosynthesis. Stronger BDG1 expression in nfxl2–1 plants may allow elevated ABA accumulation under drought stress. We conclude that the NFXL2–78 protein is part of a regulatory network that integrates the biosynthesis and action of ABA, ROS, and cuticle components.
Keywords: :
The human NFX1 transcription factor was identified as a protein that binds a conserved cis-acting element, the X-box, in promoters of class II MHC genes.Citation1 Two NFX1-like genes are typically present in the genomes of animals and higher plants, and the Arabidopsis genes were named NFXL1 and NFXL2.Citation2,Citation3 The NFXL1 gene encodes a nuclear protein that positively affects adaptation to salt stress. In contrast, NFXL2 may prevent unnecessary stress adaptation under favorable conditions.Citation3,Citation4 The NFXL2 gene encodes three NFXL2 isoforms, termed NFXL2–78, NFXL2–97, and NFXL2–100 according to the molecular weight of the putative proteins. The loss of NFXL2 resulted in elevated ABA-levels, reduced stomatal aperture, and enhanced survival of water stress. Introduction of the NFXL2–78 isoform largely complemented the nfxl2–1 mutant phenotype.Citation4 The nuclear localization and the Cys-rich region suggest DNA-binding capacity of the NFXL2–78 protein.
Gene expression profiling experiments with Affymetrix ATH1 microarrays indicated altered transcript levels of genes involved in cuticle development in the nfxl2–1 mutant (data not shown). The cuticle provides a protective barrier. It minimizes water loss and increases plant resistance to both biotic and abiotic stress.Citation5 Signals from the cuticle may influence trichome and stomatal numbers in the epidermis.Citation6 Cuticles consist of two types of lipophilic compounds, namely cutin polymers and monomeric very long chain fatty acids (VLCFAs, waxes).Citation7,Citation8 Cutin forms the structural backbone of the cuticle. The waxes limit nonstomatal water loss and serve additional functions.Citation9
The cutin polymers may be actively involved in signaling of abiotic stresses.Citation10 Thus, cuticle integrity is required for water stress signaling and tolerance. The molecular basis for this connection is unknown. Cutin biosynthesis could generate signaling molecules, or cuticle-associated proteins may sense stress signals, or the physical integrity of the cuticle may be required for sensing changes in the osmotic potential of the cell.Citation10 Surprisingly, impaired cuticle integrity can be associated with a positive effect on drought tolerance. Overexpression of the SHN1 ERF/AP2 domain transcription factor (also termed WIN1) caused altered wax composition and cuticle properties.Citation11,Citation12 In spite of increased cuticle permeability, SHN1 overexpressors displayed enhanced drought tolerance and recovery.Citation13 This was postulated to be a consequence of reduced stomatal density.Citation13,Citation14 Thus, the plant cuticle is intimately associated with stress signaling and developmental pathways. The pleiotropic phenotypic changes of mutants with defects in cuticle formation may often be a consequence of deregulated regulatory pathways rather than simple physical deficiencies.
NFXL2 Suppresses BDG1 and SHINE Expression
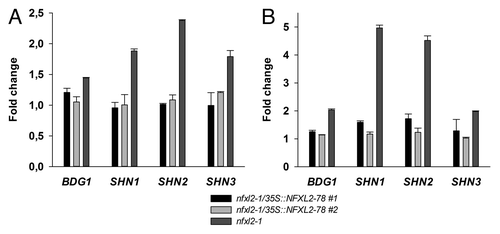
Gene expression profiling experiments with Affymetrix ATH1 microarrays indicated elevated BDG1 and SHINE transcript levels in the nfxl2–1 mutant (data not shown). Quantitative RT-PCR analysis confirmed stronger BDG1, SHN1, SHN2, and SHN3 expression in the nfxl2–1 mutant. Introduction of the NFXL2–78 coding sequence into the nfxl2–1 mutant background normalized BDG1 and SHINE expression ().
Figure 1. Quantitative RT-PCR analysis of BDG1, SHN1, SHN2, and SHN3 expression in leaves. The mean of the CT (cycle threshold) values of the reference gene (eIF1α) was subtracted from the respective CT value of the gene of interest. Subsequently, differences were subtracted from the wild-type value. Numbers give fold changes in comparison to the wild type. Error: SE of gene of interest in three technical replicates. (A) Relative transcript levels in 19-d-old plants grown in half-concentrated MS medium. (B) Relative transcript levels in 4-week-old soil-grown plants.
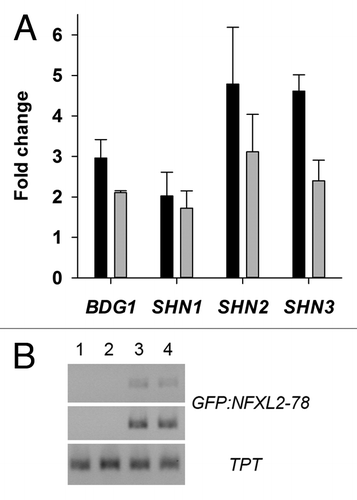
Analysis of BDG1, SHN1, SHN2, and SHN3 promoters revealed sequences with similarity to formerly identified elements with demonstrated binding of the human NFX1 protein ().Citation1,Citation15 ChIP experiments were performed with Arabidopsis wild-type plants and two independent transgenic lines expressing a green fluorescent protein (GFP)-tagged NFXL2–78 protein (35S::GFP-NFXL2–78). Both lines accumulate GFP-NFXL2–78 mRNA (), and the GFP-NFXL2–78 protein was detected in nuclei by means of confocal laser scanning fluorescence microscopy.Citation4 ChIP experiments were performed with and without the anti-GFP antibody. BDG1, SHN1, SHN2, and SHN3 promoter fragments were enriched in DNA samples of both 35S::GFP-NFXL2–78 lines probed with the anti-GFP antibody in comparison to the control experiments in which the antibody was omitted (). No enrichment was observed in wild-type samples (data not shown). Thus, the BDG1 and the SHINE genes represent direct targets of the NFXL2–78 transcription factor. However, additional experimentation is required to demonstrate binding of the NFXL2–78 protein to X-box-like promoter elements.
Figure 2. Putative X-box-like sequences in BDG1 and SHINE promoters. The X-box sequence in MHC class II promoters and the X-box-like sequence preceded by the overlapping E-box within the hTERT promoter are shown in comparison to sequences identified in the BDG1, SHN1, SHN2, and SHN3 promoters. A consensus sequence matching the depicted Arabidopsis sequences is given at the bottom. Highly conserved residues include the ACGT sequence. This sequence is part of the human E-box and resembles the core sequence of the ABA response element (ABRE).Citation20 The conserved AA and TG nucleotides were among the five residues that were shown to cause diminished NFX1–91 binding to the hTERT promoter when being mutated.Citation15 X: any nucleotide. S: C or G.
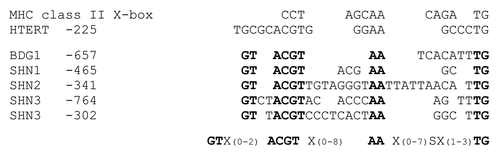
Figure 3. In vivo binding of GFP-NFXL2–78 to BDG1, SHN1, SHN2, and SHN3 promoters and analysis of GFP-NFXL2–78 transcript levels. (A) PCR amplification of promoter fragments after ChIP with an anti-GFP antibody in leaf extracts of 4-week-old soil-grown plants. Numbers give fold changes ± SE in three technical replicates (two nfxl2–1/35S::GFP-NFXL2–78 lines plus vs. minus anti-GFP antibody). (B) Semiquantitative RT-PCR was used to determine GFP-NFXL2–78 mRNA levels in 4-week-old soil-grown plants. As an internal control, the same cDNAs were used to quantify TPT transcript levels. The GFP-NFXL2–78 and TPT transcripts were amplified by PCR for 24/27 and 24 cycles, respectively. The PCR products were separated on a 1.5% agarose gel. 1, wild type; 2, nfxl2–1; 3, 35S::GFP-NFXL2–78 #1; 4, 35S::GFP-NFXL2–78 #2.
Alterations of Epidermis Properties in the nfxl2–1 Mutant
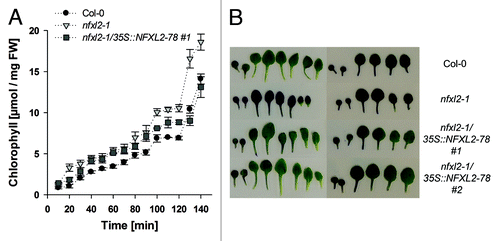
SHN1, SHN2, and SHN3 overexpression caused increased cuticle permeability.Citation13 The most widely used indicators of cuticle permeability are the elution rate of chlorophyll from leaves and staining with toluidine-blue.Citation16-Citation19 The release of chlorophyll from submerged wild-type and nfxl2–1 leaves in 80% ethanol was measured every 10 min during a period of 140 min. The chlorophyll concentration in the solution was determined. Chlorophyll loss was faster from nfxl2–1 leaves in comparison to the wild type (). Expression of the NFXL2–78 isoform in the nfxl2–1 mutant reduced chlorophyll loss (). The initial chlorophyll concentration in wild-type, nfxl2–1, and nfxl2–1/35S::NFXL-78 plants was identical (data not shown).
Figure 4. Analysis of cuticle permeability. (A) Chlorophyll leaching assays with mature rosette leaves. Rosette leaves of 4-week-old soil-grown plants were immersed in 80% ethanol for different time periods. Results are given as mean ± SE (B) Toluidine-blue staining of rosette leaves. Representative rosette leaves are shown after staining with an aqueous solution (0.05%) of toluidine blue. Left: Plants grown under aseptic conditions. Right: Plants grown in soil.
Leaf cuticle defects are rapidly visualized by toluidine-blue staining.Citation19 Leaves of the nfxl2–1 mutant showed stronger staining in comparison to wild-type plants. The complemented mutant (nfxl2–1/35S::NFXL2–78) was at a similar level to the wild type ().
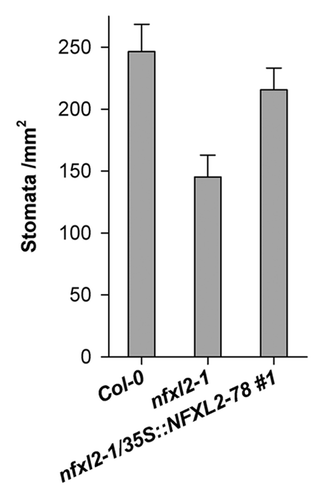
Despite an increase in cuticle permeability, SHN1 overexpression enhanced drought tolerance and recovery. This was attributed to a reduced stomatal density.Citation13,Citation14 In line with the elevated SHN1 transcript levels (), stomatal density of nfxl2–1 leaves was reduced (). Thus, stronger SHN1 expression may contribute to the enhanced drought resistance of the nfxl2–1 mutant via a reduction of stomatal density.
Figure 5. Stomatal density of mature abaxial leaf blades. Stomatal density was determined in mature rosette leaves of 4-week-old soil-grown plants. Results are given as mean ± SE. Stomatal density of nfxl2–1 plants is significantly different from the wild type (t test, p < 0.01).
SHN1 overexpression confers additional changes in the epidermal cell differentiation. 35S::SHN1 plants hardly formed trichomes on the surface of the first and second pairs of true leaves.Citation13 In agreement with stronger SHINE expression, the trichome number was significantly reduced in leaves of nfxl2–1 plants grown under standard greenhouse conditions (data not shown).
Thus, 35S::SHN1 and nfxl2–1 plants have several phenotypic changes in common, including increased cuticle permeability, lower stomatal density, enhanced drought tolerance, and lower trichome number. In contrast to 35S::SHN1 plants and the shn gain-of-function mutant,Citation11,Citation13,Citation14 the nfxl2–1 mutant was neither characterized by deep shiny green appearance, nor curled leaves, nor stunted growth.Citation4 The comparatively mild phenotypic changes of the nfxl2–1 mutant presumably are due to the moderate increase of SHINE transcript levels ().
BDG1 (also termed CED1) and further proteins involved in cutin biosynthesis are essential for osmotic stress induction of ABA biosynthesis and osmotic stress tolerance.Citation10 Binding of the NFXL2–78 protein to the BDG1 promoter may repress BDG1 expression ( and ). Stronger BDG1 expression in the nfxl2–1 mutant may allow ABA accumulation and enhanced osmotic stress tolerance.Citation10
NFXL2 is Part of a Regulatory Network with Manifold Input Signals
NFXL2–78 suppresses ABA and hydrogen peroxide accumulation, controls stomatal aperture, and mediates weaker expression of stress-related genes.Citation4 Here we have shown altered cuticle properties and elevated BDG1 and SHINE transcript levels in the nfxl2–1 mutant. Stronger SHN1, SHN2, and SHN3 expression in the nfxl2–1 mutant () presumably causes altered cuticle properties and associated effects ( and ), and the reduced stomatal density may partly account for the reduced transpiration rate of the nfxl2–1 mutant.Citation4
The multifaceted phenotypic changes of the nfxl2–1 mutant suggest that NFXL2 is not only involved in a single regulatory pathway. NFXL2 may represent a component in a network of interacting processes, in which global changes can emanate from multiple sites. Input signals include, but may not be limited to, ABA, ROS, and cuticle components. The molecular basis of their interplay is poorly understood. Analysis of the mode of action of NFXL2 may provide new insights into the complex regulatory network of drought resistance.
Materials and Methods
Growth conditions
Plants were established in soil. Seeds were allowed to germinate and grew for two weeks in controlled growth chambers (7 d: 16 h light, 140 µmol m−2 sec−1, 20°C, 75% relative humidity; 8 h night, 6°C, 75% relative humidity; thereafter 7 d: 8 h light, 140 µmol m−2 sec−1, 20°C, 60% relative humidity; 16 h night, 16°C, 75% relative humidity). Subsequently, plants used for chlorophyll leaching, toluidine-blue staining, and quantification of stomata number were transferred to long day conditions in a greenhouse with artificial light (16 h light, 21°C, 50% relative humidity; 8 h night, 19°C, 50% relative humidity). Plants used for ChIP and gene expression experiments were transferred to long day conditions in a phytotron with artificial light (16 h light, 20°C, 60% relative humidity; 8 h night, 16°C, 75% relative humidity). All genotypes were grown side by side in a randomized manner. Alternatively, plants were grown under aseptic conditions as described before.Citation4
Gene expression analysis and ChIP
Sequences of primers used for real-time RT-PCR analysis were as follows: BDG1_fw AGC TAC GGC GTC AAG AGG AA, BDG1_rev TGG CAA CCA CAA AAG AAA CA (At1g64670), SHN1_fw GTC ATG ACG GTG GAG CTA GG, SHN1_rev TTC TTC TCT GCT GCC ACC AA (At1g15360), SHN2_fw GGC TAG GAA CAT TCG ACA CG, SHN2_rev CAT CTG CAT CGC CAT TTG AT (At5g11190), SHN3_fw AGT GTG GCT TGG AAC TTT CG, and SHN3_rev TTT GGT GAC ATC AAC GGA GA (At5g25390). The eIF1α gene was used to normalize the expression levels.Citation4
Sequences of primers used for semiquantitative RT-PCR were as follows: EGFP_fw ACA TGG TCC TGC TGG AGT TC, NFXL2_rev CGC GCT CCC TGC AAG GTG GAC, TPT_fw GCT GCT TCT CAA TTC ATT ATG GGA C, and TPT_rev TGT CTG TGT CGA TAT CTT GTT TCC G.
Primers used for ChIP were as follows: BDG1_SN CCT AAG GAA GGA ACC GCA CA, BDG1_AS TCC GGT GAT GAT CCA AAA TG, SHN1_SN CCA CTG AAC AAA GTC CCA AC, SHN1_AS TGT CAA TCC AAT CCC CAA GA, SHN2_SN CCC CAT GAC ACG GAA GAG AT, SHN2_AS CTG CTT TTC CCA CGT CCT CT, SHN3_SN CGA CCC ACC GAC ATT TCT TT, and SHN3_AS AAG GCC ACC TCG TAA GTT CG. ChIP was performed using the EpiTect ChIP One-Day Kit (SABiosciences/Qiagen) and an anti-GFP antibody (Roche Applied Science) according to the manufacturer’s instruction.
Chlorophyll leaching and toluidine-blue staining
Chlorophyll leaching experiments were performed as described in Aharoni et al.Citation13 Toluidine-blue staining was performed for 5 min (plants grown under aseptic conditions) or 8 h (plants grown in a greenhouse) as described in Tanaka et al.Citation19
| Abbreviations: | ||
| ABA | = | abscisic acid |
| ChIP | = | chromatin immunoprecipitation |
| NFXL2 | = | NFX1-LIKE2 |
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to CM (MU 1738/6–1).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Song Z, Krishna S, Thanos D, Strominger JL, Ono SJ. A novel cysteine-rich sequence-specific DNA-binding protein interacts with the conserved X-box motif of the human major histocompatibility complex class II genes via a repeated Cys-His domain and functions as a transcriptional repressor. J Exp Med 1994; 180:1763 - 74; http://dx.doi.org/10.1084/jem.180.5.1763; PMID: 7964459
- Müssig C, Schröder F, Usadel B, Lisso J. Structure and putative function of NFX1-like proteins in plants. Plant Biol (Stuttg) 2010; 12:381 - 94; http://dx.doi.org/10.1111/j.1438-8677.2009.00303.x; PMID: 20522174
- Lisso J, Altmann T, Müssig C. The AtNFXL1 gene encodes a NF-X1 type zinc finger protein required for growth under salt stress. FEBS Lett 2006; 580:4851 - 6; http://dx.doi.org/10.1016/j.febslet.2006.07.079; PMID: 16905136
- Lisso J, Schröder F, Fisahn J, Müssig C. NFX1-LIKE2 (NFXL2) suppresses abscisic acid accumulation and stomatal closure in Arabidopsis thaliana. PLoS One 2011; 6:e26982; http://dx.doi.org/10.1371/journal.pone.0026982; PMID: 22073231
- Shepherd T, Wynne Griffiths D. The effects of stress on plant cuticular waxes. New Phytol 2006; 171:469 - 99; http://dx.doi.org/10.1111/j.1469-8137.2006.01826.x; PMID: 16866954
- Bird SM, Gray JE. Signals from the cuticle affect epidermal cell differentiation. New Phytol 2003; 157:9 - 23; http://dx.doi.org/10.1046/j.1469-8137.2003.00543.x
- Nawrath C. Unraveling the complex network of cuticular structure and function. Curr Opin Plant Biol 2006; 9:281 - 7; http://dx.doi.org/10.1016/j.pbi.2006.03.001; PMID: 16580871
- Pollard M, Beisson F, Li Y, Ohlrogge JB. Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 2008; 13:236 - 46; http://dx.doi.org/10.1016/j.tplants.2008.03.003; PMID: 18440267
- Samuels L, Kunst L, Jetter R. Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol 2008; 59:683 - 707; http://dx.doi.org/10.1146/annurev.arplant.59.103006.093219; PMID: 18251711
- Wang Z-Y, Xiong L, Li W, Zhu J-K, Zhu J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011; 23:1971 - 84; http://dx.doi.org/10.1105/tpc.110.081943; PMID: 21610183
- Broun P, Poindexter P, Osborne E, Jiang C-Z, Riechmann JL. WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc Natl Acad Sci U S A 2004; 101:4706 - 11; http://dx.doi.org/10.1073/pnas.0305574101; PMID: 15070782
- Kannangara R, Branigan C, Liu Y, Penfield T, Rao V, Mouille G, et al. The transcription factor WIN1/SHN1 regulates Cutin biosynthesis in Arabidopsis thaliana. Plant Cell 2007; 19:1278 - 94; http://dx.doi.org/10.1105/tpc.106.047076; PMID: 17449808
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004; 16:2463 - 80; http://dx.doi.org/10.1105/tpc.104.022897; PMID: 15319479
- Yang J, Isabel Ordiz M, Jaworski JG, Beachy RN. Induced accumulation of cuticular waxes enhances drought tolerance in Arabidopsis by changes in development of stomata. Plant Physiol Biochem 2011; 49:1448 - 55; http://dx.doi.org/10.1016/j.plaphy.2011.09.006; PMID: 22078383
- Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev 2004; 18:2269 - 82; http://dx.doi.org/10.1101/gad.1214704; PMID: 15371341
- Kurdyukov S, Faust A, Nawrath C, Bär S, Voisin D, Efremova N, et al. The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 2006; 18:321 - 39; http://dx.doi.org/10.1105/tpc.105.036079; PMID: 16415209
- Schnurr J, Shockey J, Browse J. The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 2004; 16:629 - 42; http://dx.doi.org/10.1105/tpc.017608; PMID: 14973169
- Cominelli E, Sala T, Calvi D, Gusmaroli G, Tonelli C. Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. Plant J 2008; 53:53 - 64; http://dx.doi.org/10.1111/j.1365-313X.2007.03310.x; PMID: 17971045
- Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y, Machida Y. A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 2004; 37:139 - 46; http://dx.doi.org/10.1046/j.1365-313X.2003.01946.x; PMID: 14675439
- Yamaguchi-Shinozaki K, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 2005; 10:88 - 94; http://dx.doi.org/10.1016/j.tplants.2004.12.012; PMID: 15708346