Abstract
The fungal macrocyclic lactone brefeldin A (BFA) has been a useful tool in studying protein trafficking in the secretory and endocytic pathways in plant cells. The development of various GFP-tagged organelle markers expressed in transgenic plant cells has allowed dynamic study of organelles in response to BFA in living cells. Several organelles including the endoplasmic reticulum (ER), the Golgi apparatus and endosomal compartment have been shown to have visible morphological changes in response to BFA treatment, resulting in the formation of BFA-induced aggregated compartments or ER-Golgi hybrids in various plant cells. Using transgenic tobacco BY-2 cells expressing membrane-anchored yellow fluorescent protein (YFP) reporters marking Golgi apparatus or prevacuolar compartment (PVC), we have recently demonstrated that Golgi and PVC organelles have different sensitivity to BFA, where BFA at recoverable high concentrations (50 to 100 μg/ml) also induced PVC or multivesicular body (MVB) to form aggregates in plant cells. We have thus extended the BFA action to plant PVCs/MVBs, which will serve as a useful tool for studying PVC-mediated protein sorting and PVC biogenesis.
The plant secretory pathway consists of several functionally distinct membrane-bounded compartments including the endoplasmic reticulum (ER), Golgi apparatus, prevacuolar compartment (PVC) and vacuole.Citation1,Citation2 Similar to mammalian cells and yeast, PVCs in various plant cell types including tobacco BY-2 cells, Arabidopsis embryos and germinating mung bean seeds are multivesicular bodies (MVBs) that are defined by the presence of vacuolar sorting receptor (VSR) proteins.Citation3–Citation6 PVCs have been speculated as intermediate compartments that mediate protein traffic between Golgi and vacuoles in the plant secretory pathway,Citation6,Citation7 where VSR proteins mediate protein transport from Golgi to vacuole while retromer involves recycling of the receptor proteins.Citation6,Citation8 Moreover, PVCs may also serve as a late endosome for the endocytic pathway because FM4-64 uptake studies have demonstrated that PVCs play a dual roles in endocytosis and vacuolar protein transport by showing that the internalized endosomal marker FM4-64 reaches a VSR-enriched compartment.Citation2,Citation6,Citation9
The fungal macrocyclic lactone brefeldin A (BFA) has been widely used in studying protein trafficking in the secretory pathway of plant cells where the Golgi apparatus is likely the initial site in response to BFA.Citation10 In addition to blocking protein transport from ER to the Golgi apparatus,Citation11 BFA at low concentrations (2–10 µg/ml) also induced the Golgi apparatus to form ER-Golgi hybrid structures,10 BFA compartments,Citation6,Citation12–Citation14 or the loss of Golgi cisternaeCitation15 in various plant cells. Furthermore, BFA may induce the trans-Golgi network (TGN) to incorporate into the BFA compartments in Arabidopsis root cells.Citation16 However, little is known about the possible effects of BFA on PVCs/MVBs in the plant secretory pathway.
To test if BFA treatment would cause any visible changes on PVCs, we have recently made use of the two transgenic tobacco BY-2 cell lines expressing either the Golgi marker GONST1-YFPCitation6,Citation12 or the PVC marker YFP-BP-80Citation6 and studied the dynamic changes of GFP-tagged PVCs in response to BFA treatment at both confocal and ultrastructural levels.Citation17 BFA at low concentrations (5–10 µg/ml) induced the YFP-marked Golgi stacks to form both ER-Golgi hybrid structures and BFA-induced aggregates, but had little effect on the YFP-marked PVCs in transgenic BY-2 cells at both confocal and immunogold EM levels. To our surprise, BFA at recoverable high concentrations (50–100 µg/ml) caused both GONST1-YFP-marked Golgi apparatus and YFP-BP-80-marked PVC to form aggregates in a dosage-dependent and time-dependent manner.Citation17 However, BFA-induced PVC aggregates remained physically separated from the Golgi-derived aggregates, indicating the existence of distinct BFA-induced compartments in tobacco BY-2 cells.Citation17 Furthermore, BFA at these high concentrations also induced the VSR-marked PVCs to form aggregates in root tip cells of various plants including tobacco, pea, mung bean and Arabidopsis, indicating the universal response of PVCs to BFA treatment in plants. As shown in , BFA at 100 µg/ml but not 10 µg/ml induced the VSR-marked PVCs to form visible aggregates in Arabidopsis root tip cells and Arabidopsis cultured cells, but the degree of visible changes was less in the cultured cells (). Therefore, Golgi and PVC have different sensitivity in response to BFA treatment to form physically distinct BFA-induced compartments or aggregates. A recent elegant and careful ultrastructural analysis on BFA-treated (at 10−4 M or 28 µg/ml) maize root cells demonstrated that MVBs are presented within the BFA-induced compartments,Citation18 where the morphological identification of trans-Golgi network (TGN) as vesicular compartments in maize root epidermisCitation18 is also consistent with the clathrin-coated, vesicular TGN identified as an early endosome in both Arabidopsis root cellsCitation16 and tobacco BY-2 cells in recent our studies.
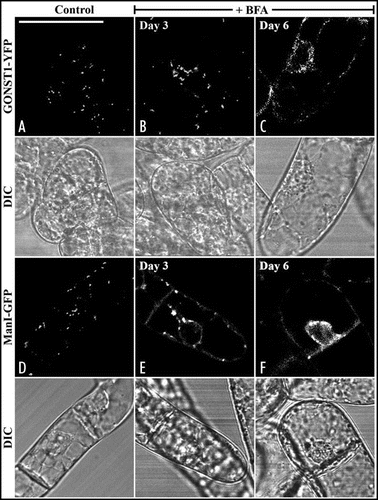
Another interesting observation from this study is that the physiological status of the cultured tobacco BY-2 cell lines may have different sensitivity to BFA. For example, when the Day-3 BY-2 cells representing log phaseCitation19 were treated with BFA at 10 µg/ml, typical BFA-induced aggregates () were observed in transgenic BY-2 cells expressing the Golgi marker GONST1-YFP, but typical ER-pattern () were observed when Day-6 cells at stationary phase were used instead for the same BFA treatment. In contrast, both Day-3 or Day-6 transgenic BY-2 cells expressing another Golgi marker Man1-GFP gave ER patterns in response to BFA treatment ( and F). It is not known if the different responses between GONST1-YFP and Man1-GFP transgenic BY-2 cell lines to BFA treatment is due to the difference between the trans-Golgi GONST1 and the cis-Golgi Man1. For example, the cis-Golgi may form ER-Golgi hybrids and proteins were return to ER, while the trans-Golgi may split from cis-Golgi to form the BFA compartments. It would thus be interesting to compare Man1 and GONST1 directly in the same cells for their dynamic response to BFA treatment in living cells, which can be achieved by coexpressing them together in transgenic BY-2 cells, a project is currently carried out in our laboratory.
To find out if the effect of BFA on the formation of PVC-derived aggregates is a primary effect on a PVC target or a secondary effect of transport from the Golgi to PVC, two phospholipase A2 inhibitor, ONO-RS-082 and bromoenol lactone (BEL), that have been used to inhibit the effect of BFA in mammalian cells,Citation20,Citation21 were also tested for their ability in preventing the formation of BFA-induced aggregates derived from either Golgi or PVC.Citation17 The obtained results demonstrated that ONO-RS-082 at 10 µM specifically prevented the formation of BFA-induced PVC aggregates, but this drug did not block the formation of BFA-induced Golgi aggregates in transgenic BY-2 cells.Citation17 Therefore, it is likely that BFA acts on distinct targets of Golgi and PVC respectively, even though its molecular mechanisms of action on PVCs remain unknown in plant cells. Such conclusion is further supported by the notable observation that VSR-marked aggregated PVCs in BFA-treated BY-2 cells seem to loss their internal vesicles, typical feature of MVBs, after BFA treatment,Citation17 a unique observation in tobacco BY-2 cells that was not observed in yeast. It thus seems that MVB biogenesis in plant cells may have alternative mechanisms distinct from yeast and mammalian cells that need further experimental data.Citation22
In conclusion, BFA is a wonderful tool for studying protein trafficking and organelle dynamics in the plant secretory pathway. Furthermore, BFA can also induce the less-characterized endosomal compartments to form BFA-induced compartments that also contain the internalized endosomal marker FM4-64.Citation23–Citation27 Therefore, BFA-induced compartments are complicated and distinct organelles originated from different organelles of the secretory and endocytic pathways in response to BFA treatments at various concentrations, which need to be better defined functionally and structurally in various plant cells in future research. BFA is thus likely a key tool in future study to dessert the cross-talk between the secretory and endocytic pathways in plant cells.
Figures and Tables
Figure 1 The formation of BFA-induced aggregates derived from VSR-marked PVCs is cell-type dependent in Arabidopsis. Arabidopsis root tips and Arabidopsis cultured cells were treated with BFA at 0, 10 and 100 µg/ml for 1 hr as indicated before they were fixed and labeled with VSRat-1 antibodies to detect PVCs. DIC = differential interference contrast images showing the morphology of the cells. Bar = 50 µm.
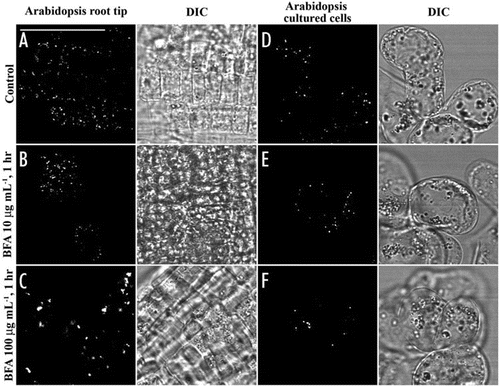
Figure 2 The formation of BFA-induced Golgi aggregates in transgenic tobacco BY-2 cells expressing either GONST1-YFP or Man1-GFP is stages dependent. Day-3 and Day-6 transgenic tobacco BY-2 cells expressing either GONST1-YFP (B and C) or Man1-GFP (right panel) were treated with BFA at 10 µg/ml for 1 hr before confocal image collection. The patterns of fluorescent signals before BFA treatments in these two transgenic cell lines were also shown (A and D). DIC = differential interference contrast images showing the morphology of the tested cells. Bars = 50 µm.
Addendum to:
References
- Jiang L, Rogers JC. Sorting of lytic enzymes in the plant Golgi apparatus. Annual Plant Review 2003; 9:114 - 140
- Lam SK, Tse YC, Jiang L, Oliviusson P, Heinzerling O, Robinson DG. Samaj J, Baluska F, Menzel D. Plant prevacuolar compartments and endocytosis. Plant Cell Monographs: Plant Endocytosis 2005; Heidelberg Springer 37 - 61
- Li YB, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh GY, Jiang L. BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol 2002; 43:726 - 742
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L. Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 Cells. Plant Physiol 2006; 142:945 - 962
- Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA. The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 2006; 18:2567 - 2581
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 2004; 16:672 - 693
- Bethke PC, Jones RL. Vacuoles and prevacuolar compartments. Curr Opin Plant Biol 2000; 3:469 - 475
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 2006; 18:1239 - 1252
- Sohn EJ, Kim ES, Zhao M, Kim SJ, Kim H, Kim YW, Lee YJ, Hillmer S, Sohn U, Jiang L, Hwang I. Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell 2003; 15:1057 - 1070
- Nebenfuhr A, Ritzenthaler C, Robinson DG. Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol 2002; 130:1102 - 1108
- Jiang L, Rogers JC. Integral membrane protein sorting to vacuoles in plant cells: Evidence for two pathways. J Cell Biol 1998; 143:1183 - 1199
- Baldwin TC, Handford MG, Yuseff MI, Orellana A, Dupree P. Identification and characterization of GONST1, a golgi-localized GDP-mannose transporter in Arabidopsis. Plant Cell 2001; 13:2283 - 2295
- Satiat-Jeunemaitre B, Cole L, Bourett T, Howard R, Hawes C. Brefeldin A effects in plant and fungal cells: Something new about vesicle trafficking?. J Microsc 1996; 181:162 - 177
- Wee EG, Sherrier DJ, Prime TA, Dupree P. Targeting of active sialyltransferase to the plant Golgi apparatus. Plant Cell 1998; 10:1759 - 1768
- Hess MW, Muller M, Debbage PL, Vetterlein M, Pavelka M. Cryopreparation provides new insight into the effects of brefeldin A on the structure of the HepG2 Golgi apparatus. J Struct Biol 2000; 130:63 - 72
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006; 18:715 - 730
- Tse YC, Lo SW, Hillmer S, Dupree P, Jiang L. Dynamic response of prevacuolar compartments to brefeldin A in plant cells. Plant Physiol 2006; (in press)
- Hause G, Samaj J, Menzel D, Baluska F. Fine structural analysis of brefeldin A-induced compartment formation after high-pressure freeze fixation of maize root epidermis. Plant Signaling and Behavior 2006; 1:134 - 139
- Matsuoka K, Demura T, Galis I, Horiguchi T, Sasaki M, Tashiro G, Fukuda H. A comprehensive gene expression analysis toward the understanding of growth and differentiation of tobacco BY-2 cells. Plant Cell Physiol 2004; 45:1280 - 1289
- de Figueiredo P, Brown WJ. Clofibrate inhibits membrane trafficking to the Golgi complex and induces its retrograde movement to the endoplasmic reticulum. Cell Biol Toxicol 1999; 15:311 - 323
- de Figueiredo P, Drecktrah D, Katzenellenbogen JA, Strang M, Brown WJ. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc Natl Acad Sci USA 1998; 95:8642 - 8647
- Jiang L, Erickson A, Rogers J. Multivesicular bodies: A mechanism to package lytic and storage functions in one organelle?. Trends Cell Biol 2002; 12:362 - 367
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003; 112:219 - 230
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001; 413:425 - 428
- Samaj J, Baluska F, Voigt B, Schlicht M, Volkmann D, Menzel D. Endocytosis, actin cytoskeleton, and signaling. Plant Physiol 2004; 135:1150 - 1161
- Ueda T, Uemura T, Sato MH, Nakano A. Functional differentiation of endosomes in Arabidopsis cells. Plant J 2004; 40:783 - 789
- Ueda T, Yamaguchi M, Uchimiya H, Nakano A. Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. Embo J 2001; 20:4730 - 4741