Abstract
Plants have evolved exquisite ways to detect their enemies and are able to induce defenses responses tailored to their specific aggressors. Insect eggs deposited on a leaf represent a future threat as larvae hatching from the egg will ultimately feed on the plant. Although direct and indirect defenses towards oviposition have been documented, our knowledge of the molecular changes triggered by egg deposition is limited. Using a whole-genome microarray, we recently analyzed the expression profile of Arabidopsis thaliana leaves after oviposition by two pierid butterflies. Eggs laid by the large white Pieris brassicae modified the expression of hundreds of genes. The transcript signature included defense and stress-related genes that were also induced in plants experiencing localized cell death. Further analyses revealed that cellular changes associated with a hypersensitive response occur at the site of egg deposition and that they are triggered by egg-derived elicitors. Our study brings molecular evidence for previous observations of oviposition-induced necrosis in other plant species and might illustrate a direct defense of the plant against the egg. In this addendum, we discuss the relevance of the oviposition-induced gene expression changes and the possibility that plants use eggs as cues to anticipate their enemies.
Plants respond to herbivorous insects by producing antidigestive proteins, toxic secondary metabolites, and enzymes whose activity is detrimental to insect growth or development.Citation1,Citation2 They have also evolved sophisticated indirect defenses that employ the release of volatile compounds to attract natural predators of their enemies. Insect eggs constitute another threat for the plant as they give rise to hatching larvae that feed on the host. Early egg recognition and activation of anti-egg or anti-insect mechanisms could thus provide an efficient defense response. Indeed, both direct and indirect responses to oviposition have been reported in plants.Citation4 For example, induced growth of neoplasm moves weevil eggs away from the pea pod surface, increasing the risk of desiccation or predation.Citation5 Rice plants produce benzyl benzoate that kills eggs of the white-backed planthopper.Citation6 Twigs of Pinus sylvestris emit volatiles after oviposition by the pine sawfly Diprion pini, attracting the egg parasitoid Chrysonotomyia ruforum.Citation7 However, although there is evidence that plants are capable of detecting the presence of insect eggs, there is almost no information on the molecular changes that take place in the host following oviposition.
In our study, we investigated the response of Arabidopsis thaliana to oviposition by two different pierid butterflies using whole-genome microarrays. Pieris brassicae egg mass triggered large changes in gene expression, with up to 671 induced genes and 426 repressed genes 72 h after oviposition. Pieris rapae, a species laying only one egg per site, caused a similar although much weaker response. Surprisingly, a comparison with the transcript signature of Arabidopsis plants challenged with chewing larvae of the specialist P. rapae revealed very little overlap between oviposition- and herbivory-induced genes. In contrast, oviposition by P. brassicae triggered a transcriptional response similar to the hypersensitive response (HR) caused by the bacterial pathogen Pseudomonas syringae AvrRPM1, or to expression changes in acd2-2, a mutant showing spontaneous spreading lesions. Notably, egg deposition by P. brassicae caused the upregulation of classical HR marker genes, including pathogenesis-related genes PR1, PR2, PR3, PR4 and PR5, and regulators of innate immunity EDS1, PAD4, and SAG101. Genes related to programmed cell death included the anti-apoptosis BAX-INHIBITOR-1, BONZAI1 (BON1), BON1-associated protein (BAP1) and two metacaspases.
Arabidopsis leaves do not reveal any physical damage at the oviposition site as P. brassicae eggs are glued without apparent modification of the leaf epidermis. However, when we analyzed known cellular changes associated with HR, cells directly below the oviposition site stained strongly with trypan blue indicating that they were undergoing cell death. We also observed an accumulation of callose and the production of hydrogen peroxide at the oviposition site. Callose deposition is associated with lesion formation in response to pathogen invasionCitation8 and with lesions found in lesion-mimic mutants.Citation9 Hydrogen peroxide is often produced as a result of external biotic and abiotic stimuli and has been shown to play a role in the control of HR.Citation10
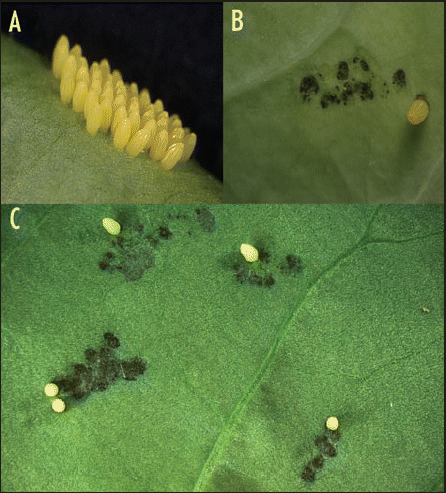
HR is an induced response triggered by the specific recognition of bacterial pathogens, viruses, fungi, and nematodes and is characterized by a localized cell death at the site of infection that prevents the progression of the disease.Citation11 Before our study, there were only two reports of egg-induced HR-like response in plants. The development of a necrotic zone at the site of egg deposition was observed in Brassica nigra resulting in egg desiccation and mortality.Citation12 This effect was only observed in some members of the plant population, illustrating a genetic basis for this mechanism. A hybrid clone of potato plant responded in a similar manner to oviposition by the Colorado beetle Leptinotarsa decemlineata. A necrotic zone containing the egg mass developed and detached from the leaf, provoking the detachment and the fall of the eggs on the soil and indirectly causing a reduction in larval survival rate.Citation13 Although we could not see a macroscopic change after P. brassicae oviposition on Arabidopsis leaves, we identified a strong response in Brassica oleracea and in rocket (Eruca sativa) plants (). Browning of leaf epidermis at the site of oviposition developed over a period of three to four days, although it did not stop egg development and larvae eventually hatched.
Collectively, these findings provide evidence that plants have the capacity to recognize eggs and that they trigger a response that has the attributes of a classical HR, both at the phenotypical and molecular levels. Microbial-induced HR is the results of a specific interaction between a pathogen avirulence gene product and a plant resistance gene. Although it is tempting to draw a parallel between pathogen- and egg-triggered HR, the identification of specific egg elicitors and the corresponding plant receptors will be required to confirm this hypothesis. Phenotypical analysis of different Arabidopsis ecotypes might reveal some genetically based variation in the response to egg deposition. Another feature of an HR is that it restricts the spread of the pathogen in the leaf. In the case of oviposition, it remains to be seen whether the HR-like response affects the development and/or hatching rate of the eggs. Our finding that proteases and lipases are induced by oviposition could indicate that these enzymes target directly the eggshell, mainly composed of proteins and wax.
In addition, oviposition by P. brassicae induced genes that are not likely to act directly against the eggs but rather against future feeding larvae. Chitinases might degrade chitin, a major component of insect cuticle and peritrophic matrices of the gut epithelium, which is absent from eggshell.Citation14 Lectins and proteases inhibitors are known to have some anti-insect properties, and tryptophan biosynthesis genes produce a precursor for several defense compounds like camalexin and indole glucosinolates.Citation15,Citation16 Moreover, egg deposition induced two terpene cyclases, indicating that synthesis of volatiles to attract predators might occur. Thus, our data suggest that, upon detection of egg elicitors, the plant could anticipate feeding larvae by inducing anti-insect defenses.
Eggs are often associated with microorganisms.Citation17 We attempted to isolate bacteria from egg extracts and could only recover three colonies that grew on LB medium, none of which being able to induce PR1:GUS expression. It is thus unlikely that microorganisms are the cause for the observed cell death response. However, we found that oviposition induced 41 receptor-like kinase (RLK) genes. RLKs are known to play an important role in the detection of pathogen-associated molecular patterns (PAMPs) like the bacterial flagellin and protein EF-Tu.Citation18,Citation19 These PAMPs induce a common transcriptional response, illustrating a general surveillance mechanism at the plant cell surface.Citation19 Interestingly, half of the egg-induced RLKs are also induced by flagellin and EF-Tu.Citation19 We can thus speculate that the transcriptional response of the plant to egg deposition is a consequence of the detection of several signals, some originating from the egg itself and responsible for the direct defense responses, and others derived from egg-associated PAMPs. The plant could interpret these PAMPs as an indication of the presence of potentially pathogenic bacteria and might thus activate RLKs as a first line of defense, waiting for other pathogenicity factors to activate more specific defenses responses.
In conclusion, we have provided molecular evidence for the detection of egg deposition by Arabidopsis plants. Future studies might show whether the HR-like induced response constitutes a direct defense against the eggs and whether plants have evolved a mechanism to anticipate the threat posed by hatching larvae.
References
- Karban R, Baldwin IT. Induced responses to herbivory 1997; University of Chicago Press
- Walling LL. The myriad plant responses to herbivores. J Plant Growth Regul 2000; 19:195 - 216
- Dicke M, van Poecke RMP, de Boer JG. Inducible indirect defence of plants: From mechanisms to ecological functions. Basic Appl Ecol 2003; 4:27 - 42
- Hilker M, Meiners T. Early herbivore alert: Insect eggs induce plant defense. J Chem Ecol 2006; 32:1379 - 1397
- Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy SR, Clement SL, Williamson RT, Carney JR, DeVilbiss ED. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc Natl Acad Sci USA 2000; 97:6218 - 6223
- Seino Y, Suzuki Y, Sogawa K. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (HORVATH) (Homoptera: Delphacidae). Appl Entomol Zool 1996; 31:467 - 473
- Hilker M, Kobs C, Varma M, Schrank K. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J Exp Biol 2002; 205:455 - 461
- Koga H, Zeyen RJ, Bushnell WR, Ahlstrand GG. Hypersensitive cell-death, autofluorescence, and insoluble silicon accumulation in barley leaf epidermal-cells under attack by Erysiphe graminis f sp hordei. Physiol Mol Plant Pathol 1988; 32:395 - 409
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL. Arabidopsis mutants simulating disease resistance response. Cell 1994; 77:565 - 577
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994; 79:583 - 593
- Kombrink E, Somssich IE. Andrews JH, Tommerup IC. Defense responses of plants to pathogens. Advances in Botanical Research 1995; Academic Press 1 - 34
- Shapiro AM, Devay JE. Hypersensitivity reaction of Brassica nigra L (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera, Pieridae). Oecologia 1987; 71:631 - 632
- Balbyshev NF, Lorenzen JH. Hypersensitivity and egg drop: A novel mechanism of host plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 1997; 90:652 - 657
- Merzendorfer H, Zimoch L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J Exp Biol 2003; 206:4393 - 4412
- Glawischnig E, Hansen BG, Olsen CE, Halkier BA. Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc Natl Acad Sci USA 2004; 101:8245 - 8250
- Wittstock U, Halkier BA. Glucosinolate research in the Arabidopsis era. Trends Plant Sci 2002; 7:263 - 270
- Kellner RL. Hilker M, Meiners T. The role of microorganisms for eggs and progeny. Chemoecology of insect eggs and egg deposition 2002; Blackwell Publishing 149 - 167
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 2006; 18:465 - 476
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006; 125:749 - 760