Abstract
The tentpole is a unique structure of the female gametophyte in Ginkgo biloba; however, its exact functions in the reproductive process are unclear. In the present study, we used semi-thin sectioning and electron microscopy to study the structure and function of the tentpole during fertilization in G. biloba. The tentpole was always initiated between two or more deeply immersed archegonia. Before fertilization, the tentpole had developed into a column-like structure, protruding toward the archegonial chamber; cells at the periphery of tentpole were loosely ranged, and abundant lipid droplets and starch grains were accumulated in the tentpole cells. After fertilization, the tentpole degenerated, and some membranous debris was overlaid on its surface. In addition, there were significant decreases in the lipids and starch grains. These results suggested that the tentpole led to the degeneration of the megaspore membrane and then supported the pliable apex of the nucellar tissues. Importantly, the tentpole also contributed to supplying nutrition for fertilization and embryo development.
Introduction
Ginkgo biloba L. is a unique living representative of the order Ginkgoales, which may have originated in the Permian approximately 150–200 million years ago.Citation1 The Ginkgoales once flourished in the early Mesozoic, but they were largely destroyed during the last ice age, with only one species, Ginkgo biloba L., surviving in China. Thus, G. biloba is believed to be one of the most primitive extant seed plants.Citation2 Many botanists have studied the primitive characteristics of G. biloba in terms of its morphology and anatomy. There are a number of characteristics of the reproductive process that are considered to be ancestral in G. biloba, including the structure of the archegonium, pollen chamber, tentpole, and haustorial pollen tubes.Citation3-Citation7 There has been considerable interest in the gametophyte development and fertilization process,Citation4-Citation7 while little attention has been paid to the tentpole, a unique structure in G. biloba.
The tentpole in G. biloba is commonly situated between two or more archegonia of the female gametophyte, and protrudes toward the archegonial chamber.Citation8 Researchers have speculated that the tentpole functions as a secretory structure, producing a liquid favorable for the spermatozoid to swim via its flagella toward the archegonium just before fertilization.Citation9 Our earlier research indicated that the cells of the tentpole accumulated abundant starch grains as the female gametophyte developed.Citation10 Therefore, the tentpole may play an important role in the fertilization process in G. biloba. However, to date there has been little convincing evidence about the morphology of the tentpole and its specific function in the sexual reproduction process.
In the present study, we observed the shape, anatomical structure, and ultrastructure of the tentpole in G. biloba using resin sections and scanning and transmission electron microscopy (SEM and TEM) techniques. In addition, we compared the changes in the tentpole before and after fertilization. Our findings enable deeper insights into the primitive characteristics of G. biloba, and clarify the functions of the tentpole in the reproductive process.
Results
Morphological features of the tentpole
The female gametophyte of G. biloba usually reaches the cellularization stage in approximately mid-May, and then it begins to differentiate the archegonia at the micropylar end. In many cases, some cells between the two archegonia differentiate to form the tentpole.Citation8 We observed the developmental process in detail via SEM. We observed that at approximately 55 d after pollination (Jun. 5), the center part of the micropylar end of the female gametophyte began to protrude upward to form a flat column-like structure, the early tentpole (). Cells of the tentpole actively divided and protruded gradually, although they were smaller than the surrounding cells (). Approximately 110 d after pollination, the tentpole was a cylindrical, 300-μm high structure. Cells at the base near the endosperm cells had a regular appearance (), whereas the upper cells of the tentpole were wrinkled and irregularly arranged (). At this stage, archegonia with swollen neck cells were located around the tentpole (). The tentpole was located nearly in the center of three archegonia, with similar distances between the tentpole and each archegonium. Meanwhile the archegonia sunk down into the endosperm, while the tentpole and the surrounding cells protruded upward. This formed a pattern in which the tentpole and archegonia protruded in the center, while the area surrounding them showed a concave appearance (). At approximately 120 d after pollination, the upper cells of the tentpole ceased to divide and began to enlarge (). The loosely ranged outer cells were no longer wrinkled; in contrast, they protruded outwards and had a plump appearance (). At approximately 130 d after pollination, the tentpole appeared to tilt toward one side (). At this stage the upper cells were arranged loosely (), whereas the lower cells were regularly arranged and had a wrinkled appearance (). An interesting phenomenon that was observed during this period was that a membranous substance emerged on the surface of the tentpole. It occurred either as a type of debris () or as a thinner but well-distributed layer (). At approximately 150 d after pollination, at harvest time, the embryo was well developed. Generally only one of the two or more zygotes developed to form the embryo, while the other one(s) aborted. The tentpole showed a significant inclination toward the developing embryo (). At that time, there was still a thin layer of the membranous substance overlaying the tentpole surface, but the outer cells had a wrinkled appearance ().
Figure 1. Morphological structures of G. biloba tentpole during development and fertilization. (A) Micropylar end of female gametophyte at ~55 d after pollination. (B, C) Gradually protruding tentpole consisting of small cells. (D) Cylindrical tentpole at ~110 d after pollination. (E) Irregularly shaped cells of tentpole. (F) Distribution of tentpole and archegonia. (G, H) Loosely ranged superficial cells at ~120 d after pollination. (I) Tilted tentpole at ~130 d after pollination. (J) Upper cells of the tentpole at ~130 d after pollination. (K, L) Wrinkled cells of tentpole. (M-Q) Membranous debris on tentpole surface. (R, S) Layer of membranous substance on tentpole surface. (T) Tentpole and embryo at ~150 d after pollination. (U) Tentpole surface at ~150 d after pollination. A, Archegonium; E, embryo; T, tentpole.
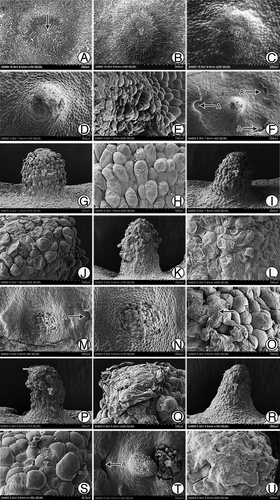
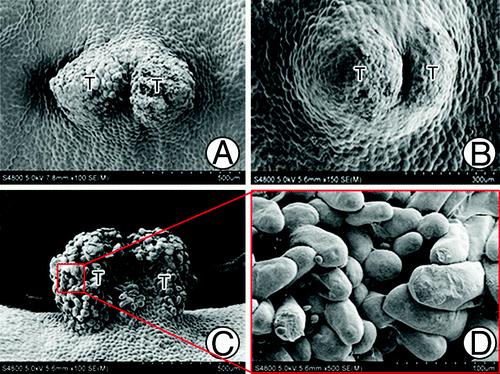
Generally, one female gametophyte had only one well-developed tentpole (). However, we observed some exceptions; show two tentpoles connected at the base that originated from one female gametophyte. show an over-developed tentpole in which nearly all the surface cells were highly specialized to form finger-like protrusions.
Figure 2. Abnormal tentpoles. (A) Two tentpoles initiated in one female gametophyte. (B) An extra tentpole originated from the base of a tentpole that had already formed. (C) Over-developed tentpole with highly specialized surface cells. (D) Magnified photo of (C), showing the finger-like surface cells. T, Tentpole.
Anatomical features of the tentpole during fertilization
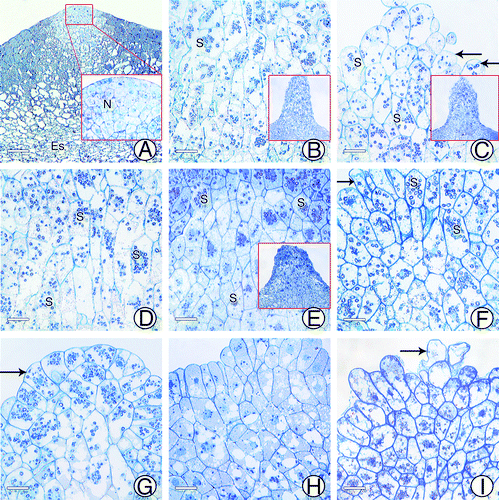
At approximately 55 d after pollination, the cells between the archegonia started to differentiate. They were approximately square-shaped and closely arranged, with large nuclei and light-stained cytoplasm (). There were no signs of nutrient accumulation, such as starch grains (). Cells at the top of the tentpole were small, but showed vigorous cell division (). At approximately 110 d after pollination, cells of the tentpole enlarged and began to accumulate starch grains. The cells had two different shapes: the inner and lower cells were elongated longitudinally, whereas the upper cells were not (). At approximately 120 d after pollination, the outer cells of the tentpole protruded outward and accumulated more starch grains, but the cytoplasm was still lightly stained (). The inner cells elongated significantly, but showed a decrease in the number of starch grains (). At approximately 130 d after pollination (the crucial fertilization period, Aug. 20) the upper cells continued to accumulate starch grains, resulting in dense staining of the cytoplasm (). The inner cells showed a reduction in starch grains, and many contained no starch grains at all, although the nuclei were still visible (). After fertilization, starch grains were only present in the upper cells, and were almost completely consumed in the inner cells. Upper cells from different seeds showed different shapes; some had finger-like protrusions (), and others were regularly arranged (). Before harvest, starch grains gradually disappeared from the upper cells, while the nuclei, some vacuoles, and densely stained materials remained visible (). At harvest time, cells had begun to collapse from the upper part of the tentpole, with only the nuclei remaining visible ().
Figure 3. Anatomical structures of tentpole during development. (A) Cells at micropylar end of female gametophyte at ~55 d after pollination. (B) Tentpole at ~110 d after pollination, with starch grains beginning to accumulate. (C) Surface cells of tentpole at ~120 d after pollination. (D) Inner cells of tentpole at ~120 d after pollination. (E) Tentpole cells at ~130 d after pollination. (F) Finger-like surface cells of tentpole. (G) Regularly arranged surface cells of tentpole. (H) Tentpole cells after fertilization. (I) Tentpole cells at harvest time. N, Nuclei; S, Starch grain. Scale Bar = 20 μm.
Ultrastructure of tentpole during fertilization
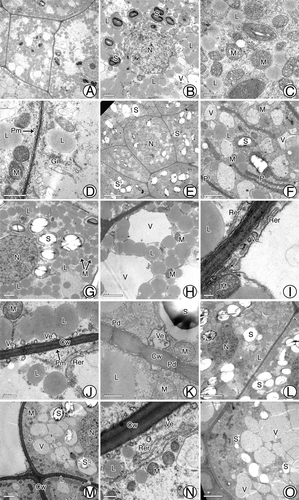
At approximately 110 d after pollination, cells of the tentpole were approximately square with a prominent nucleus (). They contained many spherical lipid droplets that were distributed densely around the vacuoles and the nucleus. At this time, starch grains began to accumulate in plastids, although these grains were very small (). There were abundant mitochondria, Golgi complexes, and small vacuoles scattered in the cytoplasm (), and the plasma membrane had a convex appearance (). At approximately 120 d after pollination (the period just before fertilization), the starch grains enlarged (), the number of vacuoles increased (), and the plastids elongated (). During fertilization, the spherical or elliptical starch grains reached their maximum volume and showed a significant hilum and layer vein. They were approximately 3 μm in diameter; therefore, they can be classified as ‘C-type’ (). There were still many lipid drops around the vacuoles (). The rough endoplasmic reticulum (RER) in the periplasmic region showed a parallel arrangement with the cell wall, with many small vacuoles and mitochondria scattered among the RER membranes (). Vesicles were present in the peripheral cytoplasm and they were frequently joined to the plasma membrane (). Moreover, there were some plasmodesmata on the cell wall (), which were likely important for material transport. After fertilization, starch grains lost their central hilum and were surrounded by vacuoles (). There was a decrease in the number and size of lipid droplets, especially near the vacuoles (), and some material appeared on the surface of the outer cell wall (). The nucleus began to degenerate (), but the mitochondria and RER were still present (). At harvest time, all organelles were difficult to distinguish, and only large vacuoles and some starch grains remained in cells. However, the layer vein of starch grains had completely disappeared ().
Figure 4. Ultrastructures of tentpole cells before and after fertilization. (A-D) Tentpole cells at ~110 d after pollination containing many mitochondria, golgi complexes, lipid droplets, plastids, and vacuoles. (E, F) Cells at ~120 d after pollination with enlarged starch grains and vacuoles. (G-K) Cells before fertilization with starch grains at maximum size, numerous vesicles and RER distributed near boundary and plasmodesmata scattered along cell wall. (L-N) After fertilization, many starch grains surrounded by vacuoles and marked decrease in lipid droplets. (O) At harvest, only large vacuoles and a few starch grains remain in cells. Cw, Cell wall; Gi, Golgi complex; L, Lipid; M, mitochondria; N, nucleus; P, Plastid; Pm, Plasma membrane; RER, Rough endoplasmic reticulum; S, Starch; V, Vacuole; Ve, Vesicle. Scale bars: (A, O, L) = 5 μm; (B, F-H, M) = 2 μm; (C-D) = 1 μm; E = 10 μm; I = 0.2 μm; (J-K, N) = 0.5 μm.
Discussion
Morphological features of tentpole that favor fertilization
In plants, there are some essential morphological features in both male and female partners that facilitate fertilization and allow successful reproduction.Citation11,Citation12 For example, in Ceratopteris thalictroides, a fertilization pore forms in the upper extra egg membrane that tightly surrounds the egg before insemination, and the spermatozoid subsequently enters through it.Citation13,Citation14 As another example, the sperm of Ceratopteris richardii extend to their full length once they are released from the antheridium, and their width decreases by approximately one-half. This allows enough space for more than one sperm to swim side by side along the canal of the egg, so as to maximize the likelihood of fertilization.Citation12 Gymnosperms also have some special architectural features to enhance the rate of fertilization success. For instance, the archegonial chamber in Encephalartos villosus provides sufficient space for the spermatozoid to swim toward its target.Citation15 The pollen tubes of Pinus monticola and Picea sitchensis may branch after arriving at the megagametophytes as a strategy to search for archegonia.Citation16 In G. biloba, just before fertilization, the proximal unbranched portion of the male gametophyte that is anchored to the pollen chamber swells and takes on a saccate appearance, characterizing maturation of the spermatozoid.Citation5 After release, the spermatozoid must swim from the pollen chamber and the archegonial chamber to the archegonium for successful fertilization.Citation17 Previous studies showed that both the pollen chamber and the archegonial chamber may undergo programmed cell death to connect to each other before fertilization.Citation3,Citation18 Therefore, the only barrier between the spermatozoid and the archegonium would be the layer of megaspore membrane that separates the nucellar tissues from the female gametophyte.Citation6 In the present study, we found many morphological and structural characteristics of the tentpole that favored entry of the spermatozoid into the archegonium. First, inside the megaspore membrane, the tentpole protruded from between two or more archegonia due to the proliferation of apical cells of the female gametophyte (). At maturity, the tentpole was approximately 300-μm high and protruded upwards (). Except for those on the surface layer, the tentpole cells were arranged compactly, suggesting that the tentpole had a firm structure. During fertilization, there was usually some membranous debris overlaying the tentpole surface. We assumed that the tentpole initiated degeneration of the megaspore membrane ( and ), and consequently assisted the spermatozoid to enter the archegonium from the archegonial chamber to complete fertilization. Second, the tentpole was usually initiated in an area central to several archegonia on a female gametophyte, in other words, the archegonia were generally located around the tentpole. Furthermore, the base of the column-like tentpole slightly protruded from the adjacent endosperm cells, while the archegonium was embedded deeply in the female gametophyte, with the portion toward the tentpole more deeply embedded and the other part protruding. Therefore, we can conclude that after the rupture of the megaspore membrane, the tentpole probably exercised its fundamental function: to hold the pliable apex of the nucellar tissues and, therefore, protect both the male gametophyte and the archegonia and prevent them from contacting each other ( and ). It could also help the pollen chamber retain its shape, thereby providing enough space for swelling of the proximal unbranched portion of the male gametophyte ().
Figure 5. Tentpole development. (A) Initiation of tentpole between archegonia. (B) Mature tentpole. (C) Tentpole is assumed to stimulate megaspore membrane. (D) Tentpole supporting apex of the nucellus. Ar, archegonium; Ac, archegonial chamber; E, egg cell; Mg, male gametophyte; Mm, megaspore membrane; Nu, nucellus; Pc, pollen chamber; T, tentpole.
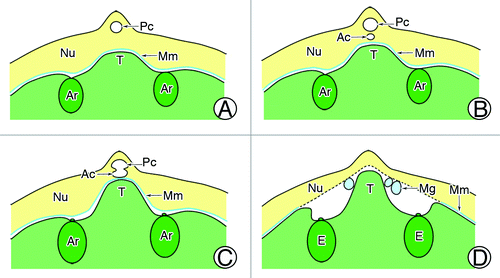
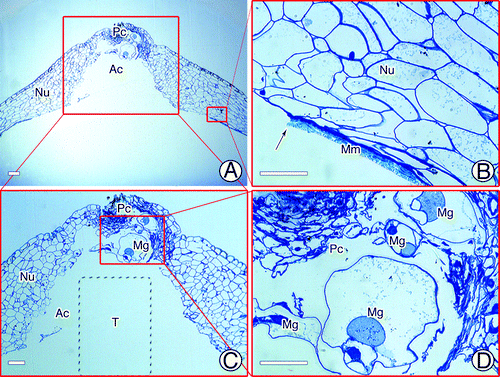
Figure 6. Apex of nucellus just before fertilization. (A, B) Rupture of megaspore membrane before fertilization. (C) Pollen chamber and archegonial chamber. (D) Four swollen male gametophytes in pollen chamber. Ac, archegonial chamber; Mg, male gametophyte; Mm, megaspore membrane; Nu, nucellus; Pc, pollen chamber. Scale bars = 50 μm.
Anatomical features of the tentpole favoring fertilization
As the fertilization period approaches, some anatomical features emerge in the female gametophyte that indicate that the male and female gametes will contact each other. In siphonogamous gymnosperms such as Pseudotsuga menziesii and Picea glauca, numerous lipid droplets accumulate in the archegonial chamber at the exact site to which pollen tubes grow. This may be an analogous situation to the chemical signaling in gymnosperm pollen tubes.Citation19-Citation21 Zoidogamous plants must produce fluid to form a pool around the archegonia. The male gamete is released into the pool and moves through it to fertilize the archegonium.Citation17,Citation22 G. biloba is one of the most notable zoidogamous gymnosperms.Citation23,Citation24 Studies on the sexual reproductive process in G. Biloba confirmed that when the spermatozoid is released from the pollen tube, the archegonial chamber is full of liquid, through which the spermatozoids swim.Citation7,Citation17 It was thought that the liquid originated from the pollen tube, or from the degenerated nucellar tissues.Citation7 In this research, we observed and analyzed the ultrastructure of the tentpole before and after fertilization. We observed that during fertilization, the loosely arranged upper cells of the tentpole protruded outwards or showed a finger-like appearance, similar to the stigma of many angiosperms during maturation.Citation25-Citation27 The cytoplasm was very dense in the upper cells of the tentpole, and they contained abundant organelles such as vacuoles, plastids, mitochondria, Golgi complexes, and RER. The mitochondria could provide considerable energy for material synthesis and transportation; the Golgi complexes would produce vesicles to participate in exocytosis; and the RER would also be important for material synthesis. In addition, some plasmodesmata were scattered along the cell walls. The presence and distribution of those cellular structures and organelles indicated the intensive exchange of material and information among the tentpole cells. We also observed the accumulation of abundant nutrients in the tentpole before and during fertilization. In the early period of tentpole formation, there were abundant lipid droplets, which remained densely distributed in cells until the time approaching fertilization. After fertilization there was a marked decrease in the size and abundance of lipid droplets. This observation suggested that lipids, which are important substances synthesized in the tentpole, participated in the fertilization process. Starch grains were another important substance synthesized in the tentpole. The plastids in tentpole cells accumulated abundant, well-developed, small starch grains before fertilization. After fertilization, the hilum and layer vein disappeared, indicating that they were exhausted during fertilization. Therefore, we can conclude that the G. biloba tentpole cells have secretory characteristics. During fertilization, the tentpole can secrete some materials that facilitate successful fertilization.
Materials and methods
Plant material
Specimens were randomly collected from healthy female G. biloba trees every week from mid-April to early October in 2009 to 2011 at the Ginkgo Experimental Station in Yangzhou University, Yangzhou, China (32°20′N, 119°30′E). The tentpoles were located at the micropylar parts of the female gametophytes in the G. biloba ovules. Therefore, after removal of three layers of seed coats, the micropylar parts were carefully dissected out from the female gametophytes with a razor blade and a steel needle under a dissecting microscope.
Scanning electron microscopy
The samples were prepared for SEM observations as follows: samples were fixed in an improved FAA solution (50% alcohol:glacial-acetic-acid:formaldehyde = 89:6:5 v/v/v) and kept at 4°C prior to use. They were dehydrated in a graded alcohol series (30, 50, 70, 80, 90, 95, and 100% v/v, 15 min each step), and further dehydrated with acetone, then immersed in isoamyl acetate for at least 30 min. After drying at critical point, the samples were coated with a layer of gold, and observed under a S-4800 field-emission SEM (Hitachi) at 15 kV accelerating voltage.Citation28
Semi-thin sections
Semi-thin sections were prepared as follows. Samples were prefixed in 2.5% (v/v) glutaraldehyde for 20 h at 4°C. After being postfixed in 1% (w/v) osmium tetroxide for 6 h at room temperature, the samples were washed three times in 0.2 M phosphate buffer (pH 7.2), dehydrated in an ethanol series (30, 50, 70, 80, 90, and 100% v/v, 15 min each step), treated twice for 30 min with propylene oxide, and then infiltrated with 1:1 propylene oxide/resin in embedding capsules overnight before being embedded in Spurr’s resin. Sections (1-µm thick) were cut with glass knives and then stained with 1% (w/v) toluidine blue O dissolved in 1% (w/v) sodium borate.Citation29 Sections were observed under a microscope (Zeiss Axioskop 40, Carl Zeiss Shanghai Company Ltd.).
Transmission Electron Microscopy
Samples were embedded in Spurr’s resin as for semi-thin sections. The samples were cut into 70-nm thick sections with a diamond knife, and then stained with 1% (w/v) uranyl acetate and 1% (w/v) lead citrate.Citation29 The top and surface cells of the tentpole were observed and photographed under a Philips Tecnai 12 TEM (JEOL Ltd, Tokyo, Japan).
Authors’ contributions
BJ and LW designed the project; DW, YL and MZ participated in SEM and TEM observations and semi-thin sectioning experiments; BJ and LW drafted the manuscript; and all co-authors participated in its editing.
Acknowledgments
We are very grateful to Prof. Peng Chen and Prof. Zhong Wang (Yangzhou University) for technical advice. This work was financially supported by the Natural Science Found of Jiangsu Province No. BK2011444, and the National Natural Science Foundation of China No. 30870436.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Brenner ED, Katari MS, Stevenson DW, Rudd SA, Douglas AW, Moss WN, et al. EST analysis in Ginkgo biloba: an assessment of conserved developmental regulators and gymnosperm specific genes. BMC Genomics 2005; 6:143; http://dx.doi.org/10.1186/1471-2164-6-143; PMID: 16225698
- Mohanta TK, Occhipinti A, Atsbaha Zebelo S, Foti M, Fliegmann J, Bossi S, et al. Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS One 2012; 7:e32822; http://dx.doi.org/10.1371/journal.pone.0032822; PMID: 22448229
- Li DH, Yang X, Cui KM, Lee CL. Morphological changes in nucellar cells undergoing programmed cell death (PCD) during pollen chamber formation in Ginkgo biloba.. Acta Bot Sin 2003; 45:53 - 63
- Gifford EM, Lin J. Light microscope and ultrastructural studies of the male gametophyte in Ginkgo biloba: the spermatogenous cell. Am J Bot 1975; 62:974 - 81; http://dx.doi.org/10.2307/2441642
- Friedman WE. Growth and development of the male gametophyte of Ginkgo biloba within the ovule (in vivo). Am J Bot 1987; 74:1797 - 815; http://dx.doi.org/10.2307/2443963
- Ji CJ, Yang X, Lee CL. Cytological studies on the formation of female gametophyte and development of archegonia in Ginkgo biloba.. Acta Sci Nat Univ Pek 1999; 35:496 - 502
- Ji CJ, Aniwar M, Fang JY. Current status on female gametophyte and fertilization in Ginkgo biloba.. Acta Bot Boreal Occident Sin 2003; 23:158 - 63
- Wang L, Jin B, Lin MM, Lu Y, Teng NJ, Chen P. Studies of the development of female reproductive organs in Ginkgo biloba L. Chin Bull Bot 2009; 44:673 - 81
- Wang L, Lu Y, Jin B, Lin MM, Chen P. Gametophyte development and embryogenesis in Ginkgo biloba: a current view. Chin Bull Bot 2010; 45:119 - 27
- Lu Y, Wang L, Pan Y, Chen P, Wang D, Xie Y, et al. Research on starch and protein accumulation and metabolism during the development of the Ginkgo biloba female gametophyte. Acta Hortic Sinica 2011; 38:15 - 24
- Russell SD. Fertilization in angiosperms. In: Pua EC, Davey MR, eds. Plant developmental biology–biotechnological perspectives. Berlin: Springer Berlin Heidelberg 2010; 283–300.
- Lopez–Smith R, Renzaglia K. Sperm cell architecture, insemination, and fertilization in the model fern, Ceratopteris richardii.. Sex Plant Reprod 2008; 21:153 - 67; http://dx.doi.org/10.1007/s00497-008-0068-x
- Cao JG, Yang NY, Wang QX. Ultrastructure of the mature egg and fertilization in the fern Ceratopteris thalictroides.. J Integr Plant Biol 2009; 51:243 - 50; http://dx.doi.org/10.1111/j.1744-7909.2008.00780.x; PMID: 19261067
- Cao JG, Wang QX, Bao WM. Formation of the fertilization pore during oogenesis of the fern Ceratopteris thalictroides.. J Integr Plant Biol 2010; 52:518 - 27; http://dx.doi.org/10.1111/j.1744-7909.2010.00942.x; PMID: 20590982
- Steyn EMA, Strydom DJE, Botha A. Fertilization and rejection of spermatozoids by egg cells in artificially pollinated ovules of Encephalartos (Zamiaceae). Sex Plant Reprod 1996; 9:175 - 85; http://dx.doi.org/10.1007/BF02221398
- Dumont–BéBoux N. Weber M, Ma Y, von Aderkas P. Intergeneric pollen–megagametophyte relationships of conifers in vitro. Theor Appl Genet 1998; 97:881 - 7
- Lee CL. Fertilization in Ginkgo biloba.. Bot Gaz 1955; 117:79 - 100; http://dx.doi.org/10.1086/335894
- Li DH, Yang X, Cui KM. Formation of archegonium chamber is associated with nucellar-cell programmed cell death in Ginkgo biloba.. Protoplasma 2007; 231:173 - 81; http://dx.doi.org/10.1007/s00709-007-0257-8; PMID: 17762908
- Takaso T, Owens JN. Postpollination–prezygotic ovular secretions into the micropylar canal in Pseudotsuga menziesii (Pinaceae). J Plant Res 1996; 109:147 - 60; http://dx.doi.org/10.1007/BF02344540
- Runions CJ, Owens JN. Sexual reproduction of interior spruce (Pinaceae). I. Pollen germination to archegonial maturation. Int J Plant Sci 1999; 160:631 - 40; http://dx.doi.org/10.1086/314170
- Rafińska K, Bednarska E. Localisation pattern of homogalacturonan and arabinogalactan proteins in developing ovules of the gymnosperm plant Larix decidua Mill. Sex Plant Reprod 2011; 24:75 - 87; http://dx.doi.org/10.1007/s00497-010-0154-8; PMID: 21069390
- Pettitt JM. The megaspore wall in gymnosperms: ultrastructure in some zooidogamous forms. Proc R Soc Lond B Biol Sci 1977; 195:497 - 515; http://dx.doi.org/10.1098/rspb.1977.0023
- Poort RJ, Visscher H, Dilcher DL. Zoidogamy in fossil gymnosperms: The centenary of a concept, with special reference to prepollen of late Paleozoic conifers. Proc Natl Acad Sci U S A 1996; 93:11713 - 7; http://dx.doi.org/10.1073/pnas.93.21.11713; PMID: 16594095
- Gorelick R, Olson K. Is lack of cycad (Cycadales) diversity a result of a lack of polyploidy?. Bot J Linn Soc 2011; 165:156 - 67; http://dx.doi.org/10.1111/j.1095-8339.2010.01103.x
- Ciampolini F, Faleri C, Di Pietro D, Cresti M. Structural and cytochemical characteristics of the stigma and style in Vitis vinifera L. var. sangiovese (Vitaceae). Ann Bot (Lond) 1996; 78:759 - 64; http://dx.doi.org/10.1006/anbo.1996.0186
- Kuta E, Bohdanowicz J, Słomka A, Pilarska M, Bothe H. Floral structure and pollen morphology of two zinc violets (Viola lutea ssp. calaminaria and V. lutea ssp. westfalica) indicate their taxonomic affinity to Viola lutea.. Plant Syst Evol 2012; 298:445 - 55; http://dx.doi.org/10.1007/s00606-011-0557-5
- Suárez C, Castro AJ, Rapoport HF, Rodríguez-García MI. Morphological, histological and ultrastructural changes in the olive pistil during flowering. Sex Plant Reprod 2012; 25:133 - 46; http://dx.doi.org/10.1007/s00497-012-0186-3; PMID: 22476326
- Jin B, Wang L, Wang J, Teng NJ, He XD, Mu XJ, et al. The structure and roles of sterile flowers in Viburnum macrocephalum f. keteleeri (Adoxaceae). Plant Biol (Stuttg) 2010; 12:853 - 62; http://dx.doi.org/10.1111/j.1438-8677.2009.00298.x; PMID: 21040300
- Jin B, Wang L, Wang J, Jiang KZ, Wang Y, Jiang XX, et al. The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana.. BMC Plant Biol 2011; 11:35; http://dx.doi.org/10.1186/1471-2229-11-35; PMID: 21329528