Abstract
Arabidopsis plants flower in response to long days (LDs). Exposure of leaves to inductive day lengths activates expression of FLOWERING LOCUS T (FT) protein which moves to the shoot apical meristem (SAM) to induce developmental reprogramming. SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) and FRUITFULL (FUL) are induced by FT at the apex. We previously screened the SAM for mRNAs of genes required to promote the floral transition in response to photoperiod, and conducted detailed expression and functional analyses on several putative candidates. Here, we show that expression of AGAMOUS-LIKE 24 (AGL24) is detected at the SAM under SD conditions and increases upon exposure to LDs. Mutations in AGL24 further delay flowering of a soc1 ful double mutant, suggesting that flowering is controlled by AGL24 partly independently of SOC1 and FUL.
Flowering of Arabidopsis is controlled by endogenous and environmental factors to ensure it occurs at the most appropriate time of year. Day length, or photoperiod, is one of the most reliable indicators of seasonal time and plants have evolved sophisticated networks to monitor its yearly progression. Day length is perceived in the phloem of leaves that produce a mobile signal partly encoded by FLOWERING LOCUS T (FT). Upon exposure to long days (LDs), the FT protein moves to the shoot apical meristem and activates the signaling cascade that converts the vegetative meristem into a reproductive meristem.Citation1 Overexpression of FT under a viral constitutive promoter (CaMV 35S), under a meristem-specific promoter (KNAT1) or under a phloem-specific promoter (SUC2) activates flowering at the shoot apical meristem (SAM) independently of photoperiodic induction, and induces flower formation also under short day lengths (SDs).Citation2,Citation3 At the apex, FT activates transcription of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) and FRUITFULL (FUL).Citation4-Citation7 Consistently, recent data demonstrated that early flowering caused by ectopic expression of FT under a constitutive or phloem-specific promoter is strongly suppressed by a soc1 ful double mutant.Citation8,Citation9 The inductive effects of SOC1 and FUL at the SAM are antagonized by the SHORT VEGETATIVE PHASE (SVP) floral repressor,Citation10 because the soc1–2 ful-2 svp-41 triple mutant can partly restore earlier flowering of the soc1–2 ful-2 double mutant.Citation9,Citation11 SVP has a dual repressive role in leaves and at the SAMCitation11-Citation13. In leaves, it directly represses FT transcription, whereas in the SAM it directly represses SOC1 transcription.Citation12,Citation13 The early flowering phenotype observed in soc1–2 ful-2 svp-41 is likely not caused by increased FT transcription in leaves, because overexpression of FT from the SUC2 promoter cannot completely rescue late flowering of the soc1–2 ful-2 double mutant.Citation9 These data suggest the existence of additional genes whose expression is required at the SAM to promote flowering in parallel to SOC1 and FUL.
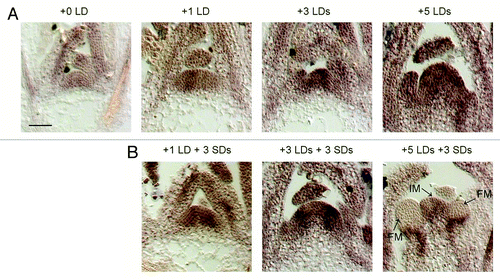
We screened candidate genes that could act with SOC1 and FUL and we focused on AGAMOUS-LIKE 24 (AGL24) for several reasons. First, by screening our data set of genes differentially expressed at the SAM during floral transition, we observed increasing AGL24 expression upon photoperiodic induction, similarly to SOC1 and FUL expression.Citation9 However, as opposed to SOC1 and FUL, AGL24 increase is relatively modest and expression can already be detected in the vegetative meristem prior to induction by LDs. Additionally, agl24 mutants are late flowering compared with wild-type controls, both under SD and LD conditions, but retain sensitivity to photoperiod.Citation14,Citation15 We conducted a detailed expression analysis by in situ hybridizations on SAMs grown for 2 weeks under SDs and then shifted to 1, 3 and 5 LDs. The results confirmed that expression of AGL24 can be detected in non-induced SAMs and progressively increases at the apex and in young leaf primordia upon exposure to inductive LDs (). We also assayed AGL24 expression on apices of plants returned to SDs after the LD treatments. After 3 additional days of growth under SD conditions we still detected AGL24 expression in the SAM (), suggesting that its expression is maintained independently of day length. A similar behavior was observed for FUL expression, particularly in samples committed to flowering (after 5 LDs), but not for SOC1 expression.Citation9 Apices harboring floral meristems after 5 d of induction under LDs + 3 SDs showed AGL24 expression in the undifferentiated inflorescence meristem and at the base of developing floral meristems (). FT mRNA expression is induced in leaves of plants transiently exposed to LDs, and is downregulated as soon as plants are returned to SD conditions.Citation16 Therefore, AGL24 expression does not follow the pattern of FT expression across a SD-LD-SD double shift.
Figure 1. Expression pattern of AGL24 in response to photoperiod. (A) In situ hybridizations of AGL24 on apices of wild-type Columbia grown for 2 weeks in SDs (0 LDs) and then transferred to LDs for one, three or five days. (B) Analysis of AGL24 expression by in situ hybridizations after transient exposure of SD-grown plants to LDs. Plants were grown for two weeks in SDs, transferred to LDs and then back to SDs as indicated. Samples were harvested at ZT8. The in situ probe spans the 3′ end and 3′ UTR regions of the AGL24 transcript, and has been described in.Citation14 For hybridization methods see.Citation9 IM: inflorescence meristem; FM: floral meristem. Bar = 50μm.
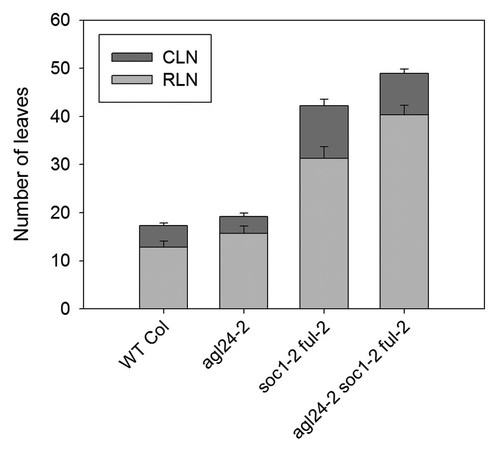
To assess if AGL24 could genetically act in parallel to SOC1 and FUL we crossed the soc1–2 ful-2 double mutant with agl24–2 to generate a triple mutant. We scored flowering time under inductive LDs and observed that mutations in agl24 can delay flowering of a soc1–2 ful-2 mutant by around 9 rosette leaves (). Taken together, these data suggest that AGL24 is required to promote the floral transition, in parallel to SOC1 and FUL. Interestingly, removing a functional AGL24 gene from the soc1 ful background has a similar effect to removing FLOR1, a gene identified by transcriptomic analyses.Citation9
Figure 2.agl24–2 enhances the late-flowering phenotype of soc1–2 ful-2 double mutants. Flowering time of plants grown under LDs. CLN, cauline leaf number; RLN, rosette leaf number. Error bars represent standard deviation. At least 8 plants were used to score flowering time of each genotype.
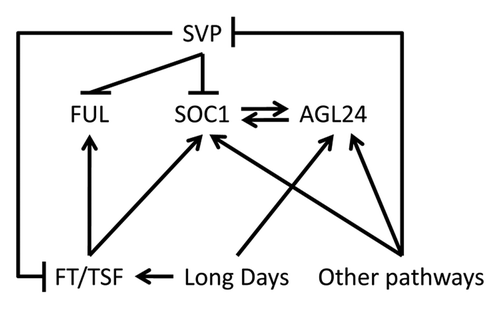
Upon floral induction, the SAM receives inductive signals from several pathways and diverse inputs have to converge into flower development programs. SOC1, FUL, SVP and AGL24 can act as floral integrator genes that respond to several environmental and endogenous cuesCitation12,Citation17,Citation18 (). Notably, all genes belong to the MADS-box family of transcription factors, a group of regulators that play important roles during floral transition and subsequent flower development. Tight regulation of their expression by the environment and cross regulation between them ensures stable progression of the floral transition.Citation12,Citation19 The photoperiodic pathway mediates information from day length to floral promoters such as FT and its close paralog TWIN SISTER OF FT (TSF).Citation20 However, FT and TSF might not be the only signals produced upon exposure to LDs. Exposure to LDs leads to increased expression of AGL24 at the apex, possibly indicating that AGL24 is a target of FT or TSF. However, promotion of flowering by ectopic expression of FT is strongly suppressed in a soc1-2 ful-2 double mutant, bearing a functional AGL24 gene. We speculate that AGL24 might not be acting downstream of FT at the apex, because in that case it would be expected that FT ectopic expression in soc1-2 ful-2 could activate AGL24 transcription and promote flowering, bypassing the requirement for SOC1 and FUL. Since both FT-dependent and FT-independent pathways can lead to activation of gene expression at the apex and influence flowering,Citation9 AGL24 expression could be enhanced by LDs but be insensitive to induction by FT (). Alternatively, upregulation of AGL24 by FT could be mediated by SOC1, that can directly promote AGL24 mRNA expression.Citation15,Citation19 However, consistent with an FT- and SOC1-independent regulation of AGL24 is (i) the fact that AGL24 expression is activated at the apex also under SDs, when FT expression in leaves is very low or absent and SOC1 is not expressed at the apex, and (ii) expression is stably maintained after returning plants to SD conditions after LD induction.
Figure 3. Genetic interactions occurring at the SAM during floral induction. Arrows represent transcriptional activation. Perpendicular lines indicate transcriptional repression.
SOC1, AGL24 and FUL proteins were shown to physically interact with each other in a yeast-two-hybrid screen.Citation21 Functional data suggest that interaction of AGL24 with SOC1 is required to carry SOC1 protein to the nucleus,Citation22 indicating that the molecular function of AGL24 depends, at least partly, on SOC1. No functional data about the interaction of AGL24 with FUL have been presented to date. Our genetic analyses indicate that the function of AGL24 does not completely depend on SOC1 and FUL, because in that case a soc1–2 ful-2 agl24–2 mutant should not flower later than soc1–2 ful-2. The existence of multiple complexes containing MADS-box proteins at different developmental stages and cell types represents a further layer of complexity overlaid on the genetic pathways that lead to flowering. Elucidating this layer of regulation will be required to improve our understanding of flowering.
| Abbreviations: | ||
| FT | = | FLOWERING LOCUS T |
| TSF | = | TWIN SISTER OF FT |
| SAM | = | shoot apical meristem |
| AGL24 | = | AGAMOUS-LIKE 24 |
| LD | = | long days |
| SD | = | short days |
| SOC1 | = | SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 |
| FUL | = | FRUITFULL |
Acknowledgments
We are grateful to George Coupland for critical reading of the manuscript. Fabio Fornara is supported by ERC starting Grant #260963.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 2008; 59:573 - 94; http://dx.doi.org/10.1146/annurev.arplant.59.032607.092755; PMID: 18444908
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, et al. Activation tagging of the floral inducer FT. Science 1999; 286:1962 - 5; http://dx.doi.org/10.1126/science.286.5446.1962; PMID: 10583961
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 2004; 131:3615 - 26; http://dx.doi.org/10.1242/dev.01231; PMID: 15229176
- Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, et al. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 2006; 20:898 - 912; http://dx.doi.org/10.1101/gad.373506; PMID: 16600915
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, et al. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol 2005; 139:770 - 8; http://dx.doi.org/10.1104/pp.105.066928; PMID: 16183837
- Teper-Bamnolker P, Samach A. The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell 2005; 17:2661 - 75; http://dx.doi.org/10.1105/tpc.105.035766; PMID: 16155177
- Schmid M, Uhlenhaut NH, Godard F, Demar M, Bressan R, Weigel D, et al. Dissection of floral induction pathways using global expression analysis. Development 2003; 130:6001 - 12; http://dx.doi.org/10.1242/dev.00842; PMID: 14573523
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat Genet 2008; 40:1489 - 92; http://dx.doi.org/10.1038/ng.253; PMID: 18997783
- Torti S, Fornara F, Vincent C, Andrés F, Nordström K, Göbel U, et al. Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. Plant Cell 2012; 24:444 - 62; http://dx.doi.org/10.1105/tpc.111.092791; PMID: 22319055
- Hartmann U, Höhmann S, Nettesheim K, Wisman E, Saedler H, Huijser P. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J 2000; 21:351 - 60; http://dx.doi.org/10.1046/j.1365-313x.2000.00682.x; PMID: 10758486
- Jang S, Torti S, Coupland G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J 2009; 60:614 - 25; http://dx.doi.org/10.1111/j.1365-313X.2009.03986.x; PMID: 19656342
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, et al. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell 2008; 15:110 - 20; http://dx.doi.org/10.1016/j.devcel.2008.05.002; PMID: 18606145
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev 2007; 21:397 - 402; http://dx.doi.org/10.1101/gad.1518407; PMID: 17322399
- Yu H, Xu Y, Tan EL, Kumar PP. AGAMOUS-LIKE 24, a dosage-dependent mediator of the flowering signals. Proc Natl Acad Sci U S A 2002; 99:16336 - 41; http://dx.doi.org/10.1073/pnas.212624599; PMID: 12451184
- Michaels SD, Ditta G, Gustafson-Brown C, Pelaz S, Yanofsky M, Amasino RM. AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J 2003; 33:867 - 74; http://dx.doi.org/10.1046/j.1365-313X.2003.01671.x; PMID: 12609028
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007; 316:1030 - 3; http://dx.doi.org/10.1126/science.1141752; PMID: 17446353
- Lee J, Lee I. Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 2010; 61:2247 - 54; http://dx.doi.org/10.1093/jxb/erq098; PMID: 20413527
- Parcy F. Flowering: a time for integration. Int J Dev Biol 2005; 49:585 - 93; http://dx.doi.org/10.1387/ijdb.041930fp; PMID: 16096967
- Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han JH, et al. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 2008; 135:1481 - 91; http://dx.doi.org/10.1242/dev.020255; PMID: 18339670
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol 2005; 46:1175 - 89; http://dx.doi.org/10.1093/pcp/pci151; PMID: 15951566
- de Folter S, Immink RG, Kieffer M, Parenicová L, Henz SR, Weigel D, et al. Comprehensive interaction map of the Arabidopsis MADS Box transcription factors. Plant Cell 2005; 17:1424 - 33; http://dx.doi.org/10.1105/tpc.105.031831; PMID: 15805477
- Lee J, Oh M, Park H, Lee I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J 2008; 55:832 - 43; http://dx.doi.org/10.1111/j.1365-313X.2008.03552.x; PMID: 18466303