Abstract
Differential expression of antioxidant enzymes in various growth and differentiation stages has been documented in several plant species. We studied here, the difference in the levels of protein content and antioxidant enzymes activity at two stages of maturity, named young and mature in neem (Azadirachta indica A. Juss), pigeonpea (Cajanus cajan (L.) mill sp) and mulberry (Morus Alba L.) leaves. The results showed that detached neem and pigeonpea mature leaves possessed higher activities of catalase (CAT) and peroxidase (POD) and lower activities of polyphenol oxidase (PPO) and ascorbate peroxidase (APX) as compared with young leaves. However, glutathione reductase (GR) showed higher activity in mature leaves of neem, whereas no change in its activity was observed in pigeonpea. On the other hand, antioxidant enzymes in mulberry showed either positive (PPO) or negative (POD, GR, APX) correlation with the progression of leaf maturity. Apparently the trend of changes in antioxidant enzymes activity during leaf development is species-specific: their activity higher at mature stage in some plants and lower in others.
Introduction
The potentially reactive derivatives of oxygen, ascribed as reactive oxygen species (ROS) such as superoxide radical (O-2), hydrogen peroxide (H2O2), hydroxyl radicals (●OH), and alkoxyl radicals (RO●) are routinely generated at low levels by plant cells.Citation1 Under normal circumstances, production and destruction of ROS is well regulated in cell metabolism and there is equilibrium between the ROS generated and antioxidant enzymes present.Citation2 However, exposure of plants to adverse environmental conditions, ROS production will overcome scavenging systems favoring the ROS upsurge that culminates in oxidative stress.Citation3 In these conditions, ROS attacks vital biomolecules and disturb the cell metabolism and ultimately the cell causes its own death. Plants have developed specific antioxidative defense enzymes including catalase (CAT, EC 1.11.1.6), peroxidase (POD, EC 1.11.1.7), polyphenol oxidase (PPO, EC 1.10.3.1), ascorbate peroxidase (APX, EC 1.11.1.1) and glutathione reductase (GR, EC 1.6.4.2) to control the rapidly increasing ROS under various environmental stress conditions.Citation4
Plant species have been found to be either sensitive or moderately tolerant to stress factors although considerable variability in stress tolerance has been reported among and within plant species.Citation5 Since the activity of antioxidant enzymes appears to be species-specific, the regulation of these enzymes in various plant species is important for understanding the ROS scavenging system. Morever, how the dynamic balance between the ROS and the antioxidative enzymes might be disturbed in plants remains unclear. In addition, it is generally thought that there is switching on and off gene activity from one stage to another.Citation6 Recently, many researchers have focused on the functional aspects of antioxidative defense system. Very little is known about the antioxidative defense system in neem (Azadirachta indica A. Juss), pigeonpea (Cajanus cajan (L.) Millsp.) and mulberry (Morus Alba L.) leaves. In view of the several ethnobotanical uses of these plant species, it was proposed to screen its successive extracts for the in vitro antioxidant enzyme activity at two different stages of maturity, and to determine if the changes in antioxidant enzyme activities during leaf senescence can be considered to be species-specific.
Results
Protein content
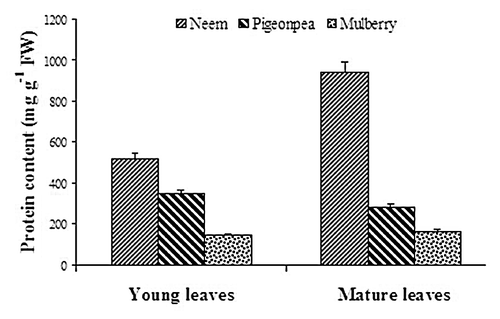
Changes in protein content were studied in neem, pigeonpea and mulberry young and mature leaves (). Protein content markedly increased from young to mature leaves in neem, whereas slight decrease in its content was detected in pigeonpea. However, there was no change in protein content was observed from young to mature leaves in mulberry. Protein content in neem mature leaves was 1.82 fold higher than young leaves, whereas in pigeonpea it was 81% of young leaves.
Antioxidative enzyme activity
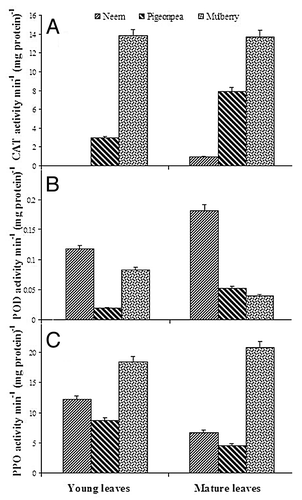
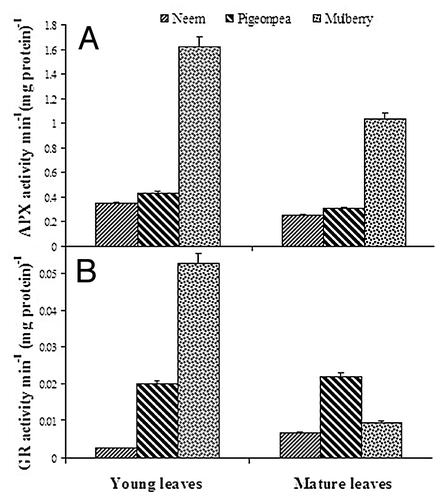
Changes in activities of antioxidant enzymes in neem, pigeonpea and mulberry leaves at two stages of maturity were studied in order to assess the role of antioxidant enzymes during leaf maturity. An increase in CAT activity was found in neem and pigeonpea leaves from young to mature leaves, whereas the activity was unchanged in mulberry (). CAT activity in mature leaves of neem and pigeonpea was 0.9 and 2.7 fold higher than young leaves, respectively. Similarly, an increase in POD activity was observed from young to mature leaves in neem and pigeonpea, but the decrease in activity was detected in mulberry (). POD activity in mature leaves of neem and pigeonpea was 1.54 and 2.78 fold higher than young leaves, respectively, whereas in mulberry the activity was 48% of young leaves. In contrast, PPO activity markedly decreased from young to mature leaves in neem and pigeonpea, whereas the activity was increased in mulberry (). PPO activity in mature leaves of neem and pigeonpea was 55% and 52% of young leaves, respectively, whereas in mulberry the activity was 1.13 fold higher than young leaves. On the other hand, APX activity markedly increased from young to mature leaves only in neem, whereas the activity was decreased in pigeonpea and mulberry (). APX activity in mature leaves of neem was 1.3 fold higher than young leaves, whereas in pigeonpea and mulberry mature leaves the activity was 60% and 66% of young leaves, respectively. However, GR activity increased from young to mature leaves in neem and pigeonpea, whereas the activity was markedly decreased in mulberry in a similar fashion as PODs did (). GR activity in mature leaves of neem and pigeonpea was 2.64 and 1.1 fold higher than young leaves respectively, whereas in mulberry the activity was 18-fold higher than young leaves.
Discussion
A number of reports are available regarding the increase and/or decrease in activities of antioxidative enzymes during various growth and differentiation stages of leaves. Differences in the protein content and antioxidant defense enzymes were reported in sunflowerCitation7 and Moringa olieferaCitation3 leaves at various growth and differentiation stages. It was reported earlier that antioxidative defense system correlate well with oxidative stress during senescence and maturity of the plants.Citation8 In present investigation, an increase in protein content from young to mature leaves in neem and mulberry suggests that young leaves may import carbon and nitrogen and rapidly undergo protein synthesis until its full capacity for protein synthesis reached. On the other hand, decreased protein content in pigeonpea mature leaves suggests that the protein turnover rate i.e., synthesis/degradation was more toward degradation in these trees.
The activity of some antioxidant enzymes studied showed higher values at the mature stage, whereas some others showed the same at young stage. CAT, APX and a variety of general PODs catalyze the breakdown of H2O2.Citation9 CAT is a tetramer of four polypeptide chains efficiently scavenges H2O2 and does not require a reducing substrate to perform the task.Citation10 The CAT activity increased from young leaves to mature leaves in neem and pigeonpea, whereas no change in its activity was detected in mulberry. Increase in CAT activity from young to mature leaves has been reported in Moringa olieferaCitation3 and sunflowerCitation7 leaves. Higher CAT activity in mature leaves might be in response to increased oxidative stress due to higher respiratory rates and energy metabolism requirement at these stages.Citation7 POD is a heam protein, which is a member of oxidoreductases and catalyzes the oxidation of a wide variety of organic and inorganic substances.Citation11 A significant increase in POD activity was reported during leaf maturity in sunflower leaves.Citation7 In this study, detection of increased POD activity in mature leaves of neem and pigeonpea could primarily be due to its role in peroxidation of cell wall polysaccharides to generate phenoxy compounds.Citation7 However, reduction of POD activity combining with the unchanged CAT activity suggests that mulberry trees might have poor H2O2 scavenging system. PPO enzyme functions as phenol oxidase in higher plants. Increase in PPO activity was reported during rice leaf senescence.Citation12 The PPO activity decreased from young leaves to mature leaves in neem and pigeonpea, whereas increased in mulberry. The increased PPO activity in mulberry leaves may give the plant an enhanced resistance to various biotic and abiotic stresses. On the other hand, decrease in PPO activity of neem and pigeonpea mature leaves giving the evidence to the statement of ConstabelCitation13 that PPOs have evolved a defensive role in only few species.
APX and GR are indespensable components of ascorbate-glutathione pathway, required to scavenge H2O2 produced mainly in chloroplasts and other cell organelles and to maintain the redox state of the cell.Citation14 Our results indicate decrease in the activity of APX in pigeonpea, neem and mulberry, from young leaves to mature leaves. The APX showed consonance with the reports found in Prunu armeniaca leaves, which showed decreased APX activity with aging.Citation15 It has been described that a decrease in apoplastic APX activity occurring during differentiation could render H2O2 available for POD activity allowing the processes of wall stiffening.Citation16 GR play a part in the control of endogenous H2O2 through an oxido-reduction cycle involving glutathione and ascorbate.Citation17 The GR was increased in neem and decreased in mulberry from young to mature leaves, whereas no change in its activity was detected in pigeonpea. Elevated concentration of glutathione is associated with the increase in oxidative stress tolerance.Citation18 However, the relationship between the activity of GR and leaf maturity, if any, is not clear.
Our results demonstrate that maturity of leaves in neem, pigeonpea and mulberry leaves is linked to complex changes observed in antioxidant enzymes. Apparently the trend of changes in antioxidant enzymes activity during leaf development is species-specific: their activity increases during the progression of maturity in some plants and decreases in others. Such a variation in response to leaf maturity could be due to the variability of plant species in producing free radicals. Both increasing and decreasing tendencies of antioxidant enzymes during maturity may have some functional significance during development of leaves.
Materials and methods
Site description
Neem, pigeonpea and mulberry trees grown in the farms of Biochemistry Department, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad in India were used. The site is located near about 10 km away from the center of Aurangabad, and the above trees are not under any specific air pollution. The site of the sample area is surrounded by hills on all directions, and characterized by a semiarid climate, with annual temperature in range from 9–40°C and mean annual precipitation of 725 mm. The main climatic characteristics of the sample site are shown in .
Table 1. Main characteristics of the sample site
Plant material
A sample of light green, thin young leaves and dark green, thick mature leaves at equal distance from twig tip was collected from each of three neem, pigeonpea and mulberry trees just before use ().
Preparation of leaf extract
Leaf Samples (0.3 g) were ground in 10 ml of 100 mM phosphate buffer (pH 7.0) using pre-chilled mortar and pestle. The phosphate buffer contained 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride and 1% polyvinylpyrrolidone. The homogenate was filtered through four layers of nylon cloth and the filtrate was centrifuged at 4°C at 17000 x g for 10 min. The supernatant was used for measurements of enzyme activity.
Determination of protein
Protein content was determined by Lowry’s method (1951) using bovine serum albumin as standard.Citation19
Catalase assay
The activity of CAT was assayed as described in Verma and Dubey, (2003) with slight modifications.Citation14 The reaction mixture in a total volume of 2 ml contained 100 mM potassium phosphate buffer (pH 7.0), 400 µl of 6% H2O2 and 100 µl leaf extract. Leaf extract was the last component to be added and the decrease in absorbance was recorded at 240 nm (extinction coefficient of 0.036 mM−1 cm−1) using a UV-Vis spectrophotometer (Jasco-V500, Japan) at 10s intervals up to 1 min. The specific activity of enzyme is expressed as µmol of H2O2 oxidized min−1 (mg protein)−1.
Peroxidase assay
The activity of POD was assayed as described in Rao et al. (1999) with slight modifications.Citation20 The reaction mixture in a total volume of 2 ml contained 100 mM potassium phosphate buffer (pH 6.5), 200 µl of 16 mM guaiacol, 20 µl of 6% H2O2 and 100 µl of leaf extract. Leaf extract was the last component to be added and the increase in absorbance was recorded at 470 nm (extinction coefficient 25.2 mM−1 cm−1) using a UV-Vis spectrophotometer (Jasco-V500, Japan) at 10 sec intervals up to 1 min. The specific activity of enzyme is expressed as µmol guaiacol oxidized min−1 (mg protein)−1.
Polyphenol oxidase assay
The activity of PPO was assayed as described in Saravanan et al. (2004) with slight modifications.Citation21 The reaction mixture in a total volume of 2 ml contained 100 mM potassium phosphate buffer (pH 7.0), 200 µl of catechol and 100 µl of leaf extract. Leaf extract was the last component to be added and the increase in absorbance was recorded at 420 nm using a UV-Vis spectrophotometer (Jasco-V500, Japan) at 10 sec intervals up to 1 min. The specific activity of enzyme is expressed as change in the absorbance of reaction mixture min−1 (mg protein)−1.
Ascorbate peroxidase assay
The activity of APX was assayed as described in Verma and Dubey (2003) with slight modifications.Citation14 The reaction mixture in a total volume of 2 ml contained 100 mM potassium phosphate buffer (pH 7.0), 500 µl of 0.2 mM ascorbic acid, 100 µl of 0.2 mM EDTA, 300 µl of 6% H2O2 and 100 µl of leaf extract. Leaf extract was the last component to be added and the decrease in absorbance was recorded at 290 nm (extinction coefficient 2.8 mM−1 cm−1) using a UV-Vis spectrophotometer (Jasco-V500, Japan) at 10 sec intervals up to 1 min. The specific activity of enzyme is expressed as µmol ascorbic acid oxidized min−1 (mg protein)−1.
Glutathione reductase assay
The activity of GR was assayed as described in Harinasut et al. (2003) with slight modifications.Citation22 The reaction mixture in a total volume of 2 ml contained 100 mM potassium phosphate buffer (pH 7.0), 50 µl of 0.2 mM NADPH, 100 µl of 0.5 mM GSSG, 100 µl of 2 mM EDTA and 100 µl of leaf extract. Leaf extract was the last component to be added and the decrease in absorbance was recorded at 340 nm (extinction coefficient 6.2 mM−1 cm−1) using a UV-Vis spectrophotometer (Jasco-V500, Japan) at 10 sec intervals up to 1 min. The specific activity of enzyme is expressed as µmol NADPH oxidized min−1 (mg protein)−1.
Statistical analysis
Each experiment was repeated three times with two replicates each and the data presented are mean values of independent experiments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol 1994; 105:467 - 72; PMID: 12232215
- Esfandiari E, Shekari F, Shekari F, Esfandiari M. The effect of salt stress on antioxidant enzymes Activity and lipid peroxidation on the wheat seedling. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2007; 35:48 - 56
- Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 2009; 64:303 - 11; http://dx.doi.org/10.1007/s11130-009-0141-0; PMID: 19904611
- Buchanan-Wollaston V. The molecular biology of leaf senescence. J Exp Bot 1997; 48:181 - 99; http://dx.doi.org/10.1093/jxb/48.2.181
- Garg N, Noor Z. Genotypic differences in plant growth, osmotic and antioxidative defense of Cajanus cajan (L.) Millsp. modulated by salt stress. Arch Agron Soil Sci 2009; 55:3 - 33; http://dx.doi.org/10.1080/03650340802393303
- Cehavir G, Yentur S, Yazgan M, Unal M, Yilmazer N. Peroxidase activity in relation to anthocyanin and chlorophyll content in juvenile and adult leaves of ‘Mini-Star’ Gazania Splendenes.. Pak J Bot 2004; 36:603 - 9
- Sairam RK, Singh DV, Srivastava GC. Changes in activities of antioxidant enzymes in sunflower leaves of different ages. Biol Plant 2003; 47:61 - 6; http://dx.doi.org/10.1023/A:1027328814591
- Zimmermann P, Zentgraf U. The correlation between oxidative stress and leaf senescence during plant development. Cell Mol Biol Lett 2005; 10:515 - 34; PMID: 16217560
- Rahnama H, Ebrahimzadeh H. Antioxidant isozymes activities in Potato plants (Solanum tuberosum L.) under salt stress. Journal of Sciences. Islamic Republic of Iran 2006; 17:225 - 30
- Kumari GT, Reddy AM, Naik ST, Kumar SG, Prasanthi J, Sriranganayakulu G, et al. Jasmonic acid induced changes in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings. Biol Plant 2006; 50:219 - 26; http://dx.doi.org/10.1007/s10535-006-0010-8
- Sat IG. The effect of heavy metals on peroxidase from Jerusalem artichoke (Helianthus tuberosus L.) tubers. Afr J Biotechnol 2008; 7:2248 - 53
- Kar RK, Choudhuri MA. Effects of light and spermine on senescence of hydrilla and spinach leaves. Plant Physiol 1986; 80:1030 - 3; http://dx.doi.org/10.1104/pp.80.4.1030; PMID: 16664713
- Constabel CP, Yip L, Patton JJ, Christopher ME. Polyphenol oxidase from hybrid poplar. Cloning and expression in response to wounding and herbivory. Plant Physiol 2000; 124:285 - 95; http://dx.doi.org/10.1104/pp.124.1.285; PMID: 10982443
- Verma S, Dubey RS. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 2003; 164:645 - 55; http://dx.doi.org/10.1016/S0168-9452(03)00022-0
- Scebba F, Sebastiani L, Vitagliano C. Activities of antioxidant enzymes during senescence of prunnus armeniaca leaves. Biol Plant 2001; 44:41 - 6; http://dx.doi.org/10.1023/A:1017962102950
- Díaz-Vivancos P, Clemente-Moreno MJ, Rubio M, Olmos E, García JA, Martínez-Gómez P, et al. Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to plum pox virus. J Exp Bot 2008; 59:2147 - 60; http://dx.doi.org/10.1093/jxb/ern082; PMID: 18535298
- EI-Shora HM. Activities of antioxidative enzymes and senescence in detached Cucurbita pepo under Cu- and oxidative stress by H2O2. Becth. Mock.. Yh-Ta 2003; 44:66 - 71
- Broadbent P, Creissen GP, Kular B, Wellburn AR, Mullineaux PM. Oxidative stress responses in transgenic tobacco containing altered levels of glutathione reductase assay. Plant J 1995; 8:247 - 55; http://dx.doi.org/10.1046/j.1365-313X.1995.08020247.x
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265 - 75; PMID: 14907713
- Rao MV, Paliyath G, Ormrod DP. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 1996; 110:125 - 36; http://dx.doi.org/10.1104/pp.110.1.125; PMID: 8587977
- Saravanan T, Bhaskaran R, Muthusamy M. Psedomonas fluorescens induced by enzymological changes in banana roots (CV.Rasthali) against Fusarium wilt disease. Plant Pathol J 2004; 3:72 - 80; http://dx.doi.org/10.3923/ppj.2004.72.80
- Harinasut P, Poonsopa D, Roengmongkol K, Charoensatapom R. Salinity effects on antioxidant enzymes in mulberry cultivar. Sci Asia 2003; 29:109 - 13; http://dx.doi.org/10.2306/scienceasia1513-1874.2003.29.109