Abstract
Cotton (Gossypium hirsutum L.) is one of the most commercially important fiber crops in the world. Compared with other crops, cotton represents a recalcitrant species for regeneration protocols. The development of efficient and rapid regeneration protocol for elite Indian cotton variety could help improve the quality characteristics and biotic or abiotic stress tolerance. Here we report a novel regeneration protocol in Indian cotton cultivar Narashima. The maximum number of multiple shoots obtained was 16 per explants, performance which has never been achieved in any prior reports. The embryo apex explants were isolated from 2 d old in vitro growing seedlings. Explants were cultured on MS medium containing different plant growth regulator combinations in order to induce multiple shoots. Among the tested combinations, the 2 mg/l of 6-benzylaminopurine (BAP) and 2 mg/l kinetin (KIN) proved to be most suited for achieving the maximum number of multiple shoots. The elongation of multiple shoots was obtained in media supplemented with gibberellic acid (GA3). The regenerated plants were successfully hardened in earthen pots after adequate acclimatization. This method avoids callus tissue, the stage of regeneration which may lead to somaclonal variation. The important feature of the presented method is shortening of regeneration time, as well as the induction of a high number of multiple shoots per explants. The present protocol may provide an efficient and rapid regeneration tool for obtaining more stable transformants from embryo apex explants of Indian cotton cultivar Narashima.
Introduction
Cotton (Gossypium hirsutum L.) is one of the most commercially important fiber crops in the world. In addition to textile manufacturing, it produces seeds with a potential multiproduct base such as hulls, oil, linters and food for animals.Citation1,Citation2 Cotton belongs to the Malvaceae family and the Gossypium genera consisting of about 50 species, from which only four (G. hirsutum, G. barbadence, G. arboreum and G. herbaceum) are domesticated and produce spinnable fiber.Citation3 G. hirsutam (upland cotton) cultivars provide the bulk of commercial cotton. Among the cotton-producing countries, India ranks first in production and cultivation area, providing 32% of the world’s total area of cotton cultivation, followed by the USA (23%) and China (20%). It has been estimated that 180 million people, directly or indirectly, depend on the production of cotton for their livelihood.Citation4
Cotton biotechnology plays a vital role in improving the quality as well as the quantity of fiber by producing plants resistant to biotic and abiotic stress. The production of plants resistant to biotic and abiotic stress through conventional breeding is limited by several factors such as lack of useful variation and the long time periods that are required. Plant biotechnology is an attractive means for improving cotton through genetic engineering. Price and SmithCitation5 (1979) first reported on biotechnological improvement in cotton (G. klotzschianum Anders.). The use of biotechnological tools, such as the biolistic method and Agrobacterium-mediated transformation, require as prerequisite suitable plant regeneration protocols that are genotype-independent, efficient and which do not yield somaclonal variant.Citation6-Citation8 In comparison to a number of other crops, cotton is more difficult to regenerate.
Unfortunately, none of the Indian cultivars could be exploited for developing transgenic cotton due to lack of efficient regeneration protocol. Therefore, there is a strong need for developing an efficient regeneration protocol involving Indian cotton cultivars with regard to multiple shooting from cotyledon explants.Citation9 There are two main types of regeneration methods for cotton biotechnology: organogenesisCitation10-Citation12 and somatic embryogenesis.Citation13-Citation15 Somatic embryogenesis studies in cotton have been previously reported.Citation13,Citation15 This method is mainly genotype-dependent and has the disadvantage of requiring a long time for reaching its purpose. It has to pass through the callus phase that may lead to chromosomal aberration or polyploidy.Citation16 However, the response is genotype-specific and may also produce somaclonal variants where a callus phase is involved.
In the present paper, we report on a new plant regeneration method for cotton. The method is an efficient, rapid, genotype-independent regeneration protocol for obtaining direct multiple shoot organogenesis from embryo apex explants with successful rooting of the regenerated shoots. This method will facilitate the application of plant tissue culture and genetic engineering in cotton.
Results and Discussion
An efficient protocol for multiple shoot regeneration in a genotype-independent manner is a pre-requisite for genetical manipulation of crop plants. Even if there is a large body of literature available on regeneration in cotton cv Coker through somatic embryogenesis, the efforts on the establishment of de novo shoot regeneration protocols in commercial cotton cultivars are rare.Citation17 In this study we have developed a highly efficient method to yield more multiple shoots in the elite cotton variety Narasimha. This variety has a practical importance since it is used as a female parental line in several conventional, transgenic or hybrid cotton.Citation18
In order to establish an efficient in vitro regeneration protocol for G. hirsutum c. narshima seeds were surface sterilized by HgCl2 before in vitro germination. This methodology has already proved to be essential in cotton tissue culture.Citation19 Cotton seed sprouting was observed in water after 24 h incubation under dark conditions. The maximum germination frequency was 80%. Different explants, such as hypocotyls, cotyledons and embryo apex, were tried for de novo regeneration. When hypocotyls and cotyledon were used, they produced callus that did not regenerate (data not shown). Embryo apex was found to be the best material for multiple shoot induction.
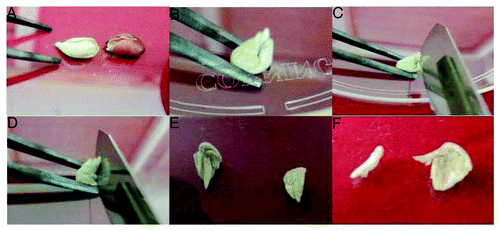
Embryo apex explants were prepared under sterile conditions. The sequential steps for the isolation of the embryo apex from cotton seeds is shown in . When embryo apex sections, 0.5–1 cm in length, were placed horizontally on the medium (), the swelled proximal end differentiated into multiple shoot buds by the end of second week (). Adventitious shoot buds and leafy structures arise from the central region and sides of the swelled proximal end of the embryo apex (). The buds further developed into individual multiple shoots (). The frequency of shoot formation was influenced by the type and concentration of the phytohormones used. The results are shown in . The highest proportion of explants forming adventitious shoots was obtained with media containing 2 mg/l BAP and 2 mg/l KIN. Lower concentrations of BAP and KIN yielded in reduced number of multiple shoots. When BAP alone was used in the media at a lower concentration (0.5 mg/l) it yielded 1.1 shoots per explants, while at higher concentration (2.5 mg/l) it yielded a maximum of 4.7 shoots (). In the same manner, the KIN used in the media at lower concentration (0.5 mg/l) yielded less shoots (1.3 per explants) while at the highest concentration (2.5 mg/l) it yielded maximum 5.5 shoots. By combining both BAP and KIN, the lower concentrations (0.5 mg/l) gave a maximum of 3.2 shoots per explants (). The maximum number of shoots per explant was 16.1, obtained by using 2.0 mg/l BAP and 2.0 mg/l KIN. At higher concentrations (2.5 mg/l), the maximum number of shoots was 11.6. So, higher concentrations of BAP or KIN negatively affected the multiplication rate of multiple bud formation, reducing the number of shootx per explants. In consequence, the cytokinin concentration plays a major role in multiple shoot induction. Cytokinin has been reported to regenerate cotton plants with short and compact shoots.Citation20
Figure 2. Cotton (Gossypium hirsutum L.) plant regeneration by organogenesis from embryo apex segments. (A) Embryo apex explants from 2-d-old seedlings on multiple shoot induction medium. (B) Two days later, embryo apex explant turned into green. (C) Swelled proximal end. (D) Multiple shoot buds. (E–I) Adventitious shoot buds and leafy structures arising from the central region and sides of the swelled proximal end of the embryo apex. (J–L) Multiple shoot bunches and their elongation.
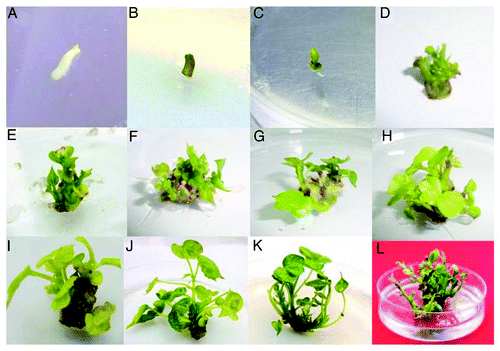
Table 1. The effect of BAP and KIN phytohormones in the induction of multiple shoot induction in cotton
In cowpea (Vigna unguiculata)Citation21 and cucumber (Cucumis sativus)Citation22, multiple shoot buds arose from the proximal end of hypocotyl explant showing that this phenomenon is common in dicotyledonous species. In the case of cotton (cv Coker 310FR), ChandharyCitation23 used hypocotyls and cotyleodonary leaf for regeneration and induction of multiple shoots. The explants were subjected to slow physical desiccation and both normal and abnormal somatic embryos were obtained. Even though an efficient regeneration protocol by somatic embryogenesis in cotton cv Narashima was reported by Khan,Citation24 the embryos were obtained from callus, a methodology that leads to somaclonal variations. In our present protocol, the callus phase is avoided, leading to the shortening of the regeneration time, as well as the induction of a high number of multiple shoots per explants. As for the culture conditions, the MS basal medium supplemented with different concentrations of BAP (from 0.5–2.5 mg/l) and KIN (from 0.5–2.5 mg/l), alone or in combination, is directly responsible for reprogramming the cotton embryonic apical meristem axes toward the multiplication of buds.Citation25 In a previous study,Citation25 the use of 3 mg/l BAP resulted in the formation of multiple shoots in cotton cv Guazuncho, with a maximum of 3.4 shoots per embryonic axis. In the protocol that we developed, a higher number of multiple shoots (~16.1 per explant) were obtained.
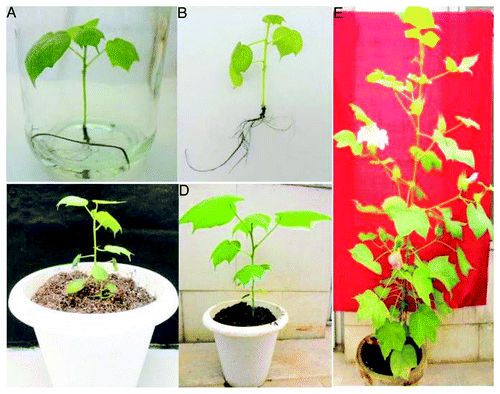
Once the multiple shoot buds were obtained, the shoot bunches were separated into individual shoots and transferred to the elongation medium, composed by MS medium supplemented with different concentration of GA3 (Gibberellic Acid) (0.5–2.0 mg/l) and cultured for 2 wk. GA3 at 1 mg/l was found to be the most suitable concentration for cotton elongation (data not shown). Subsequently, the elongated and properly developed shoots from the embryo apex explants were transferred to rooting medium containing different concentrations of IBA (0.5, 1.0, 1.5, 2.0 mg/l). The effect of auxin on in vitro root induction in cotton cv Narashima is presented in . A significant level of rhizogenesis was observed at 1 mg/l IBA and resulted into 90% rooting with the maximum root length of 7.3 ± 1.3 cm. Root induction occurred between 10–15 d after transfer to rooting medium and the rooting frequency was higher in all these explant regenerants. DivyaCitation26 reported similar results by using hypocotyl segments of cotton seedlings. In cotton tissue culture, rooting is one of the major problems and many different methods were applied to induce rooting.Citation17 However, the reports on successful rooting are very few and subsequently, further studies are still needed.
Table 2. Auxin effect on in vitro root induction and growth of cotton roots
After rooting, the plantlets were kept on MS liquid media for 1 wk in order to induce root hardening () and subsequently transferred to small pots containing a mixture of vermiculite, sand and peat moss in 1:1:1 ratio (). Each pot was covered with a polythene bag to maintain high humidity initially for the few days, after which the humidity was reduced by making holes in the polythene bags to harden the plants. So, in consequence, mature, healthy cotton plants were obtained through multiple-shoot induction starting from embryo apex.
Materials and Methods
Plant material
Delinted mature cotton seeds (G. hirsutum var Narashima) were acquired from Regional Agricultural Research Station, ANGRU (Acharya N.G. Ranga Agricultural University) Nandyal, Andhra.
Culture media and growth conditions
MS mediumCitation27 including MS vitamins, containing 3% (w/v) sucrose and 0.8% (HI-MEDIA) agar was used in all the experiments. Plant growth regulators, BAP (0.5–2.5 mg/l) and KIN (0.5–2.5 mg/l), at different concentrations were incorporated into the basal media. The pH of the medium was adjusted to 5.8 by 1 M NaOH or 1 M HCl before autoclaving at 1.06 kg cm−2 (121°C) for 20 min. The cultures were incubated at 25 ± 2°C in a culture room with 50 μmol m−2 s−1 irradiance provided by cool fluorescent lamps and were exposed to a photoperiod of 16 h and 55% relative humidity.
Preparation of explants, plant regeneration protocol and multiple shoot induction
The healthy cotton seeds were surface sterilized with 70% ethanol for 2 min and thoroughly washed with distilled water. Later the seeds were treated with 4% Bavistin (carbendazim) for 5 min, washed with water, then 5 min with sodium hypochloride (NaOCl) and finally treated with 0.1% mercuric chloride (HgCl2) for 5 min followed by washing thoroughly with sterile water 3–4 times that confirms the removal of HgCl2. All the steps above were performed under the laminar flow. The seeds were then soaked in distilled water for 48 h (until the seed coat gets opened) in the dark. Embryonic apex was isolated from 2 d (48 h) old in vitro germinated seeds by the following method: the seeds were transferred to petri plates and then pressed with forceps so that embryo is pushed out. Embryo was split longitudinally (half of its length from the top) by using a sterile scalpel. After removal of radical and primary shoots, the embryo apex is visible and isolated. The isolated embryo apex contains only the meristemal region (). The explants were cultured in a vertical upright position slightly embedded in MS medium containing B5 vitamins, 3% sucrose, 0.8% agar, with different concentrations of N-6-benzylaminopurine (BAP) and kinetin (KIN). The culture was maintained in the dark for 2 d and transferred to light conditions of 16/8 h and temperature of 25°C ± 2°C for 1 wk. The culture had to be subcultured every week.
After 3–4 wk of multiple bud inductions, the shoots were excised from the multiple shoot bunches obtained from embryo apex explant and transferred individually to culture bottles containing MS media with 1 mg/l GA3 to assess their response for elongation.
Rooting and hardening
Well-elongated shoots (2–3 cm) derived from shoot bunches of embryo apex were excised and transferred to rooting media. Rooting media consists of MS basal salts along with 0.1 mg/l Indole Butric Acid (IBA). The well-rooted plantlets need to be kept on MS liquid media for root hardening for 1 wk in the culture room. Plantlets with the hardened roots were transferred to small pots containing a mixture of vermiculite, sand and peat moss in 1:1:1 ratio. Each pot was covered with a polythene bag to maintain high humidity initially for the few days. Subsequently, the humidity was reduced by making holes in the polythene bags to harden the plants. The images were taken with the Sony NEX-3 E5 camera.
Conclusions
In conclusion, the present study reports on the development of a highly efficient regeneration protocol based on using embryo apex as explants for the induction of multiple shoots in cotton (16.1 per explant). The time taken from explanting to the establishment of plants in the greenhouse was about 3–4 mo. This time period is shorter when compared with the longer periods reported in published regeneration protocols using somatic embryogenesis. This method avoids callus tissue, the stage of regeneration which may lead to somaclonal variation. The important feature of presented method is shortening of regeneration time, as well as the induction of high number of multiple shoots per explants.
| Abbreviations: | ||
| BAP | = | 6-Benzylaminopurine |
| KIN | = | knetin |
| IBA | = | indole- 3 butyric acid |
| MS | = | Murashige and Skoog (1962) medium |
| GA3 | = | gibberlic acid |
Acknowledgments
Work on plant transformation and plant abiotic stress tolerance in NT’s laboratory is partially supported by Department of Biotechnology (DBT) and Department of Science and Technology (DST), Government of India. We thank Drs Anca Macovei, S. Raveendar and Renu Tuteja for their useful suggestions in editing the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Mishra R, Wang H, Yadav NR, Wilkins TA. Development of highly regenerable elite Acala cotton (Gossypium hirsutum cv. Maxxa) - a step towards genotype-independent regeneration. Plant Cell Tiss. Org. Cult. 2003; 73:21 - 35; http://dx.doi.org/10.1023/A:1022666822274
- Aragao FJL, Vianna GR, Carvalheira SBRC, Rech EL. Germ line genetic transformation in cotton (Gossypium hirsutum L.) by selection of transgenic meristematic cells with a herbicide molecule. Plant Sci 2005; 168:1227 - 33; http://dx.doi.org/10.1016/j.plantsci.2004.12.024
- Sakhanokho HF, Zipf A, Rajasekaran K, Saha S, Sharma GC, Chee PW. Somatic embryo initiation and germination in diploid cotton (Gossypium arboreum L.). In Vitro Cell Dev Biol Plant 2004; 40:177 - 81; http://dx.doi.org/10.1079/IVP2003497
- Benedict JH, Altman DW. Commercialization of transgenic cotton expressing insecticidal crystal protein. In J.J. Jenkins and S. Saha (Eds.) Genetic improvement of cotton: Emerging technologies. Science Publ. Enfield, NH p. 2001;137-201.
- Price HJ, Smith RH. Somatic embryogenesis in suspension cultures of Gossypium klotzschiaanum. Anderss. Planta 1979; 145:305 - 7; http://dx.doi.org/10.1007/BF00454456
- Fioozabady E, Deboer DL, Merlo DJ. Transformation of cotton (Gossypium hirsutum L.) by Agrobactium tumefaciens and regeneration of transgenic plants. Plant Mol Biol 1987; 10:105 - 16; http://dx.doi.org/10.1007/BF00016148
- Gould JH, Magallanes-Cedeno M. Adaptation of cotton shoot apex culture to Agrobacterium-mediated transformation. Plant Mol Biol Rep 1998; 16:1 - 10; http://dx.doi.org/10.1023/A:1007438104369
- McCabe DE, Martinell BJ. Transformation of elite cotton cultivars via particle bombardment of meristems. BioTechnology 1993; 11:596 - 8; http://dx.doi.org/10.1038/nbt0593-596
- Gupta SK, Singh PK, Sawant SV, Chaturvedi R, Tuli R. Effect of light intensity on in vitro multiple shoot induction and regeneration of cotton (Gossypium hirsutum L. cv Khandawa-2). Indian J Exp Biol 2000; 38:399 - 401; PMID: 11218821
- Gould J, Banister S, Hasegawa O, Fahima M, Smith RH. Regeneration of Gossypium hirsutum and G. barbadense from shoot apex tissues for transformation. Plant Cell Rep 1991; 10:12 - 6; http://dx.doi.org/10.1007/BF00233024
- Hemphill JK, Maier CGA, Chapman KD. Rapid In vitro plant regeneration of cotton (Gossypium hirsutum L.). Plant Cell Rep 1998; 17:273 - 8; http://dx.doi.org/10.1007/s002990050391
- Zapata C, Srivatanakul M, Park SH, Lee BM, Salas MG, Smith H. Improvements in shoot apex regeneration of two fiber crops: cotton and kenaf, Plant Cell Tiss. Org. Cult 1999; 54:185 - 91; http://dx.doi.org/10.1023/A:1006238924439
- Trolinder NL, Goodin JR. Somatic embryogenesis and plant regeneration in (Gossypium hirsutum L). Plant Cell Rep 1987; 6:231 - 4; http://dx.doi.org/10.1007/BF00268487
- Finer JJ. Plant regeneration from somatic embryogenic suspension cultures of cotton (Gossypium hirsutum L). Plant Cell Rep 1988; 7:399 - 402
- Firoozabady E, DeBoer DL. Plant regeneration via somatic embryogenesis in many cultivars of cotton (Gossypium hirsutum L.). In Vitro Cell Dev Biol 1993; 29:166 - 73
- Ulian EC, Smith RH, Gould J, McKnight T. Transformation of plants via the shoot apex. In Vitro Cell Dev Biol 1988; 24:951 - 4; http://dx.doi.org/10.1007/BF02623909
- Ouma JP, Young MM, Reichert NA. Rooting of in vitro regenerated cotton (Gossypium hirsutum L.) is influenced by genotype, medium composition, explant type and age. Afr J Biotechnol 2004; 36:313 - 8
- Ravindranath K. Narasimha, A versatile Gossypium hirsutum cultivar and proven parent of many superior hybrid cottons in India. J. Indian Soc. Cotton Improvement 2009; 34–37.
- Rauf S, Usman M, Fatima B, Manzoor TK, Iqrar AK. In vitro regeneration and multiple shoot induction in upland cotton (Gossypium hirsutum L.). Int. J. Agric. Biol 2004; 4:704 - 7
- Banerjee AK, Agrawal D, Nalawade SM, Krishnamurthy KV. Recovery of in vitro cotton shoots through micro grafting. Curr Sci 2000; 78:623 - 6
- Brar MS, Moore MJ, Al-Khayri JM, Morelock TE, Anderson EJ. Ethylene Inhibitors promote in vitro regeneration of cowpeas (Vigna unguiculata L.). In Vitro Cell Dev Biol Plant 1999; 35:222 - 5; http://dx.doi.org/10.1007/s11627-999-0082-1
- Mohiuddin AKM, Choudhury MKU, Abdullah ZC, Napis S. Influences of silver nitrate (ethylene inhibitor) on cucumber in vitro shoot regeneration. Plant Cell Tiss. Org. Cult 1997; 5:73 - 8
- Chaudhary B, Kumar S, Prasad KV, Oinam GS, Burma PK, Pental D. Slow desiccation leads to high-frequency shoot recovery from transformed somatic embryos of cotton (Gossypium hirsutum L. cv. Coker 310 FR). Plant Cell Rep 2003; 21:955 - 60; http://dx.doi.org/10.1007/s00299-003-0613-x; PMID: 12835904
- Khan T, Reddy VS, Leelavathi S. High-frequency regeneration via somatic embryogenesis of an elite recalcitrant cotton genotype (Gossypium hirsutum L.) and efficient Agrobacterium-mediated transformation. Plant Cell Tissue Organ Cult 2010; 101:323 - 30; http://dx.doi.org/10.1007/s11240-010-9691-y
- Jorge LM, Permingeat HR, Romagnoli MV, Heisterborg CM, Ruben HV. Multiple shoot induction and plant regeneration from embryonic axes of cotton. Plant Cell Tiss. Org. Cult 1998; 54:131 - 6; http://dx.doi.org/10.1023/A:1006170529397
- Divya K. Swathi Anuradha, Jami SK, Kirti PB. Efficient regeneration from hypocotyls explants in three cotton cultivars. Biol Plant 2008; 52:201 - 8; http://dx.doi.org/10.1007/s10535-008-0046-z
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 1962; 15:473 - 97; http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x