Abstract
VOZ (vascular plant one zinc-finger protein) is a plant specific one-zinc finger type transcriptional activator, which is highly conserved through land plant evolution. We have previously shown that loss-of-function mutations in VOZ1 and VOZ2 showed increased cold and drought stress tolerances whereas decreased biotic stress resistance in Arabidopsis. Here, we demonstrate that transgenic plants overexpressing VOZ2 impairs freezing and drought stress tolerances but increases resistance to a fungal pathogen, Colletoricum higginsianum. Consistent with changes in the tolerance to biotic and abiotic stresses, the expression of marker genes for these stresses is significantly altered compared with those of the wild-type plant. These results indicate that a overexpression of VOZ2 confers biotic stress tolerance but impairs abiotic stress tolerances in Arabidopsis.
Transcriptional regulation plays a central role in the expression of genomic information during complex biological processes in all organisms. Plants as a sessile organisms have evolved complex regulatory networks that control their responses to changes in environmental conditions in a timely manner.Citation1 Arabidopsis, a model organism of the higher plant, has a wide variety of plant specific transcription factor families such as NAC, AP2/ERF/DREB, DOF and WRKY that regulate gene expression responding to various environmental cues.Citation2
One of the plant specific transcription factor families, VASCULAR PLANT ONE-ZINC FINGER PROTEIN 1/2 (VOZ1/2) transcription factors were first isolated as binding proteins to GCGTNx7ACGC palindromic sequence in the 5′ up-stream region of V-PPase gene (AVP1) in vitro.Citation3 Recently, we have reported that the loss-of-function mutation of VOZ genes simultaneously confers abiotic stress tolerance and biotic stress susceptibility.Citation4 Furthermore, Yasui et al. have reported that VOZs regulate phytochrome B mediated flowering by controlling FT and FLC expression.Citation5 Thus, the several phenotypes of the loss-of-function mutant of VOZ genes have been shown in Arabidopsis. The phenotypes of overexpression of VOZ genes is however still largely unknown. Here we provide new insight into the biotic and abiotic stress responses in VOZ overexpression plant.
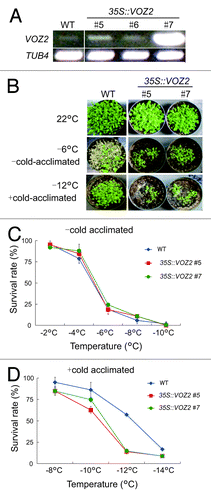
In the previous study, we demonstrated that the voz1voz2 double mutants exhibits increased drought and freezing tolerances, whereas decreased the resistance to pathogens including C. higginsianum and P. syrigae.Citation4 These results suggest that VOZs negatively regulate drought and cold-responsive pathway and positively regulate biotic-stress pathways simultaneously in Arabidopsis. To test this hypothesis, we generated transgenic plants expressing VOZ2 under control of the cauliflower mosaic virus 35S promoter. From several transgenic lines, we chose two overexpressing lines (lines #5 and #7) for further experiments (). To evaluate the effect of the overexpression in VOZ2 on abiotic stress conditions, we first performed a freezing resistance test on two VOZ2 overexpressing lines. Without prior cold acclimation, Wild-type (WT) and the VOZ2 overexpressing lines showed similar sensitivity to freezing temperatures; the survival rates of the overexpressing lines decreased drastically at temperatures below –6°C, and most of the seedlings died at -8°C (). The overexpressing lines of #5 and #7, however, were more sensitive to freezing temperatures after cold acclimation; the survival rates of the VOZ2 overexpressing plants were reduced below 25%, although the survival rate of WT was still above 50% at -12°C (). These data showed that overexpression of VOZ2 significantly reduces freezing resistance even in the cold acclimated condition.
Figure 1. Overexpression of VOZ2 reduces freezing tolerance after cold acclimation. (A) Expression level of VOZ2 in WT and several VOZ2 overexpression lines were estimated by reverse transcriptase-PCR. β-tubulin 4 was used as an internal standard. (B) Representative images of WT and 35S::VOZ2 lines before freezing treatment (upper panel), after exposure to -6°C with non-cold-acclimated (middle panel) and to -12°C for 4 h with cold-acclimated (lower panel) plants. Images were taken after a recovery growth for 7d under normal growth conditions. (C) Survival rates of WT (♦), 35S::VOZ2 #5 (■) and 35S::VOZ2 #7 (•) plants were measured under non-cold-acclimated (C) and cold-acclimated (D) conditions. Data represent means ± SD (n = 1,000 per each line).
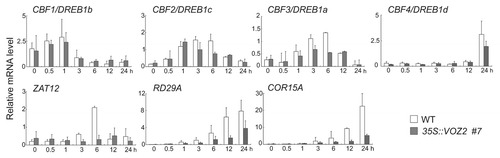
To investigate the molecular mechanism by which the gain-of-function mutation in VOZ2 affects the resistance to freezing temperatures, we compared the expression levels of several genes related to the cold-responsive pathways in the VOZ2 overexpressing line #7 with those in WT plants at 4°C using qRT-PCR (). The expression levels of a set of CBF/DREB1 (CBF1/DREB1b, CBF2/DREB1c and CBF3/DREB1a) genes were not significantly changed in the VOZ2 overexpressing plant compared with those in WT under cold-stress conditions. In contrast, the expression of a CBF/DREB1-independent cold-regulatory gene ZAT12Citation6 and that of an ABA-mediated transcription factor CBF4/DREB1dCitation7 were significantly reduced in the VOZ2 overexpressing plant under cold conditions. The expression of the cold-responsive genes COR15A, and RD29A also reduced considerably. Given the reduced expression of ZAT12, CBF4, COR15A, and RD29A, VOZ2 might negatively regulate the ABA-mediated abiotic stress responsive pathway.
Figure 2. Changes in the expression of several cold-responsive genes in the 35S::VOZ2 plant line #7 under cold-temperature conditions. The expression levels of several cold responsive genes were determined using qRT-PCR. Two-week-old plants were grown at 22°C and then incubated at 4°C for various lengths of time (0, 0.5, 1, 3, 6, 12 or 24 h). The expression level of each gene was normalized to β-tubulin4 (TUB4) expression. Data are represented as the mean ± SD (n = 3 ~4).
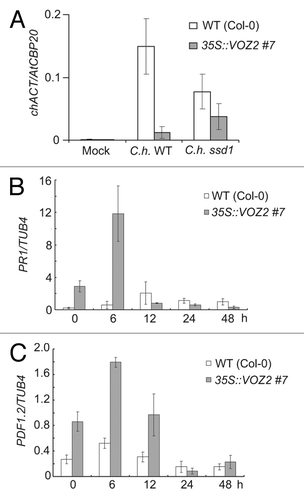
Abiotic stress tolerance has an antagonistic effect on a resistance to pathogen infections.Citation8 To investigate whether the host defense against pathogens is affected in the VOZ2 overexpressing plant, its susceptibility to the WT and the less virulent ssd1 mutant of the anthracnose fungus, Colletotrichum higginsianum, was evaluated. As shown in , endogenous contents of WT and ssd1 mutant of C. higginsianum in WT (Col-0) and 35S::VOZ2 #7 Arabidopsis cells were significantly reduced after at 7 d post inoculation (dpi), indicating that resistance to both the C. higginsianum WT and the ssd1 mutant was significantly enhanced in the VOZ2 overexpressing plants than that in WT Arabidopsis. To investigate whether SA- and JA-mediated defense responses are activated in the VOZ2 overexpressing plant, the expression levels of a SA-signaling pathway marker gene, PR1 and a JA-signaling pathway marker gene, PDF1.2 were measured using qRT-PCR after C. higginsianum infection. In WT, the PR1 and PDF1.2 expression levels increased during the first 12 h post inoculation (hpi) and then decreased over the following 24 h, whereas the expression of PR1 and PDF1.2 were upregulated even before C. higginsianum infection, and the expression of both genes were dramatically increased after 6 hpi ().
Figure 3. Infection phenotypes of WT and 35S::VOZ2 plants inoculated with C. higginsianum. (A) Quantification of C. higginsianum in plants was performed using qRT-PCR. Three-week-old plants were spray-inoculated with C. higginsianum. Inoculated leaves were harvested at 4 dpi and total RNA was isolated. qRT-PCR was performed with primers for the C. higginsianum actin gene. The data were normalized to Arabidopsis CAP-binding protein 20 gene (AtCBP20). Data are represented as the mean ± SD (n = 6). This experiment was repeated five times with similar results. (B and C) Defense response gene expression profiles in WT and 35S::VOZ2 plants. Three-week-old plants of each genotype were infected, and mRNA expression levels of PR1 (B) and PDF1.2 (C) in each genotype were measured using qRT-PCR after different lengths of time (0, 6, 12, 24, 48 h). The data were normalized to TUB4 expression. Data are represented as the mean ± SD (n = 6).
We have previously reported that the loss-of-function mutation in VOZ genes confers abiotic stress tolerances but increases biotic stress susceptibility in Arabidopsis.Citation4 Here, we demonstrated that overexpression of VOZ2 exhibits increased biotic stress resistance but decreased freezing stress tolerance phenotypes simultaneously. The results strongly support the idea in which VOZs function as a negative regulator of the abiotic stress responsive pathway and positive regulator of the biotic stress responsive pathway, controlling the adaptation of plants to various stress conditions in Arabidopsis. Future studies are needed to determine the direct targets of VOZ transcription factors in Arabidopsis.
Acknowledgments
This work was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology, a-grant-in-aid for Basic Science Research (C), and the Strategic Research Funds of Kyoto Prefectural University to M.H.S. and a grant-in-aid for Scientific Research for Plant Graduate Students from Nara Institute of Science and Technology, supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology, to Y.N., and Funding Program for Next Generation World-Leading Researchers to S.F.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 2006; 57:781 - 803; http://dx.doi.org/10.1146/annurev.arplant.57.032905.105444; PMID: 16669782
- Mitsuda N, Ohme-Takagi M. Functional analysis of transcription factors in Arabidopsis. Plant Cell Physiol 2009; 50:1232 - 48; http://dx.doi.org/10.1093/pcp/pcp075; PMID: 19478073
- Mitsuda N, Hisabori T, Takeyasu K, Sato MH. VOZ; isolation and characterization of novel vascular plant transcription factors with a one-zinc finger from Arabidopsis thaliana.. Plant Cell Physiol 2004; 45:845 - 54; http://dx.doi.org/10.1093/pcp/pch101; PMID: 15295067
- Nakai Y, Nakahira Y, Sumida H, Takebayashi K, Nagasawa Y, Yamasaki K, et al. Vascular plant one-zinc finger protein 1/2 transcription factors regulate abiotic and biotic stress responses in Arabidopsis. Plant J 2012; In press http://dx.doi.org/10.1111/tpj.12069; PMID: 23167462
- Yasui Y, Mukougawa K, Uemoto M, Yokofuji A, Suzuri R, Nishitani A, et al. The phytochrome-interacting vascular plant one-zinc finger1 and VOZ2 redundantly regulate flowering in Arabidopsis. Plant Cell 2012; 24:3248 - 63; http://dx.doi.org/10.1105/tpc.112.101915; PMID: 22904146
- Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 2005; 41:195 - 211; http://dx.doi.org/10.1111/j.1365-313X.2004.02288.x; PMID: 15634197
- Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, Zhang JZ. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 2002; 130:639 - 48; http://dx.doi.org/10.1104/pp.006478; PMID: 12376631
- Mauch-Mani B, Mauch F. The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 2005; 8:409 - 14; http://dx.doi.org/10.1016/j.pbi.2005.05.015; PMID: 15939661