Abstract
In Arabidopsis leaves there is a bi-phasic dose-response to applied nucleotides; i.e., lower concentrations induce stomatal opening, while higher concentrations induce closure. Two mammalian purinoceptor antagonists, PPADS and RB2, block both nucleotide-induced stomatal opening and closing. These antagonists also partially block ABA-induced stomatal closure and light-induced stomatal opening. There are two closely related Arabidopsis apyrases, AtAPY1 and AtAPY2, which are both expressed in guard cells. Here we report that low levels of apyrase chemical inhibitors can induce stomatal opening in the dark, while apyrase enzyme blocks ABA-induced stomatal closure. We also demonstrate that high concentrations of ATP induce stomatal closure in the light. Application of ATPγS and chemical apyrase inhibitors at concentrations that have no effect on stomatal closure can lower the threshold for ABA-induced closure. The closure induced by ATPγS was not observed in gpa1-3 loss-of-function mutants. These results further confirm the role of extracellular ATP in regulating stomatal apertures.
Extracellular ATP (eATP), which has now become recognized as a signaling agent in plants, can regulate growth in a variety of plant cell and tissue types.Citation1 The application of micromolar concentrations of ATP can induce [Ca2+]cyt fluctuations and promote growth-altering accumulation of ROS and NO in diverse tissues of diverse plants. Nitric oxide (NO) and reactive oxygen species (ROS) are important in guard cell responses. In our original study of this signaling response we found that ATPγS-induced stomatal closing was dependent on the production of ROS and NO.Citation2 Importantly we reported that both ABA and light-induced changes in stomatal aperture were preceded by an increase in the eATP levels of guard cells. A subsequent report by Hao et al. (2012)Citation3 found that applied ATP promoted stomatal opening, but, in contrast to the results we obtained using ATPγS, they did not observe stomatal closing in Arabidopsis or Vicia faba leaves in response to applied ATP. In this study we carry out additional tests that address questions raised by the findings of Hao et al. (2012),Citation3 and provide new data consistent with a proposed model for eATP regulation of stomatal aperture.
Application of 5 µM or 15 µM ATPγS in the dark induces stomatal opening,Citation2 while application of 25 µM ATPγS or more does not have an effect in the dark. Hao et al. (2012) confirmed a role for eATP in stomatal opening, showing that applied ATP at concentrations as high as 1 mM induce stomatal opening.Citation3 Regarding eATP-induced stomatal opening, we hypothesized that moderate inhibition of ectoapyrase activity by application of low concentrations of chemical apyrase inhibitors would cause naturally occurring levels of eATP to increase resulting in stomatal opening. We found that, similar to treatment with 15 μM ATPγS, treatment of leaves with two different apyrase inhibitors at a concentration of 1.5 µg/mL also induces stomatal opening ().
Figure 1. (A) Treatment with light induces stomatal opening. Treatment with 15 μM ATPγS or 1.5 μg/mL apyrase inhibitor NGXT191 or 1.5 μg/mL apyrase inhibitor #13 induces stomatal opening in the dark. (B) Treatment with 100 μM DTT blocks light-induced opening. (C) Treatment with light and treatment with 150 μM ADP in the dark induces stomatal opening in Col-0 and the gpa1–3 mutant. Apertures measured in epidermal peels as width/length after 2 h treatment of whole leaves. Different letters above bars = mean values that are significantly different from one another as determined by Student’s t-test (p < 0.05; n > 50).
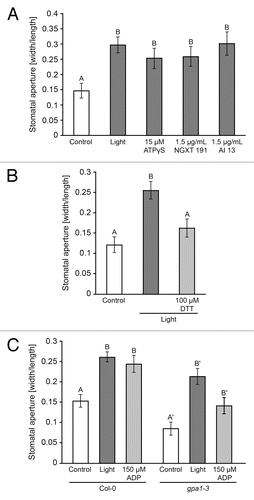
We reported that application of 150 µM ATPγS or more in the light induces stomatal closure,Citation2 but Hao et al. (2012)Citation3 did not observe stomatal closure when treating leaves with ATP. In our previous experiments we found that application of ATPγS can induce changes in plant growth at 10-fold lower concentrations than ATP, presumably because applied ATP is hydrolyzed by ectoapyrases or other phosphatases. Thus our expectation was that stomatal closure induced by applied ≥ 150 µM ATPγS would also be induced by ATP but at 10-fold higher concentrations (≥ 1.5 mM), so we performed closing experiments using ATP and found that > 1.5 mM ATP did indeed induce stomatal closure (). Interestingly, just as application of soluble potato apyrase blocked stomatal opening in the light,Citation3 we found that it could block ABA induced-closure ().
Figure 2. (A) Treatment with 10 μM ABA induces stomatal closure in the light, as did 150 μM ATPγS and 1.5 mM ATP. (B) Treatment with 10 μM ABA induces stomatal closure in the light, but in combination with 8 units of potato apyrase closure is blocked. (C) Treatment with either 0.1 μM ABA or 1.5 μg/mL apyrase inhibitor #13 alone does not change stomatal apertures, but in combination induce stomatal closure in the light. (D) Combining 10 μM ABA with 8 units of boiled apyrase has no effect on ABA-induced closure. Treatment with either 0.1 μM ABA or 75 μM ATPγS alone does not change stomatal apertures, but in combination induce stomatal closure in the light. (E) Treatment with 10 μM ABA induces stomatal closure in both Col-0 and the gpa1–3 mutant in the light, however treatment with 250 μM ATPγS in the light only induces stomatal closure in Col-0. Apertures measured in epidermal peels as width/length after 2 h treatment of whole leaves. Different letters above bars = mean values that are significantly different from one another as determined by Student’s t-test (p < 0.05; n > 50).
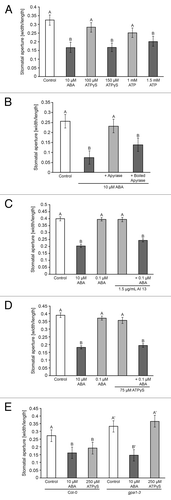
The observation that purinoceptor antagonists can partially block ABA-induced closure and that ABA treatment of leaves induces a rapid increase in eATP levels suggests that eATP is part of the ABA signal transduction pathway. In order to further test this hypothesis we examined whether eATP and ABA could act synergistically to induce stomatal closure. We found that 0.1 μM ABA alone was not enough to induce stomatal closure in our system so we combined this concentration of ABA with concentrations of apyrase inhibitor and ATPγS that also were too low alone to have an effect on stomatal closure. However, we found that combining low levels of ABA with either low levels of two different apyrase inhibitors or low levels of ATPγS resulted in stomatal closure ().
ATPγS-induced stomatal closure and ATP-induced stomatal opening require production of H2O2 by NADPH oxidase (RBOHD/F).Citation2,Citation3 Application of H2O2 induces stomatal closure in Arabidopsis,Citation4 while application of CuCl2 and ascorbic acid to introduce hydroxyl radicals induces opening at 0.1 mM, but not above 0.5 mM.Citation3 These observations suggest that the biphasic response to ATPγS may be a biphasic response to ROS, with lower amounts of ATPγS causing production of lower amounts of ROS.
In addition to ROS, ATPγS-induced closure requires production of NO by nitrate reductase, with production of ROS temporally preceding production of NO.Citation2 This is consistent with the fact that ABA-induced NO production is dependent on H2O2 production in Arabidopsis.Citation5 Furthermore, ATP-induced NO production requires phosphatidic acid (PA) production in tomato,Citation6 and PA inhibits ABI1 phosphatase activity to allow ROS production by RBOHD/F in ABA-induced closure in Arabidopsis.Citation7 This potentially places PA production upstream of ROS production in ATP-induced closure.
ATP elicits two calcium spikes in Arabidopsis root hairs, the first occurring 30 – 40 s after application and the second 80 – 90 s after application, with the first spike involving Ca2+ influx and the second release of Ca2+ from internal stores.Citation8 ATP-induced PA accumulation in tomato is not sensitive to EGTA, while ATP-induced NO production is,Citation6 placing the first Ca2+ spike downstream of PA production but upstream of NO production. During ABA- and H2O2-induced stomatal closure, H2O2 activates plasma membrane Ca2+ channels in a manner dependent on GCA2 and ABI2.Citation4,Citation9 This places the first Ca2+ spike downstream of H2O2. Finally, NO-induced stomatal closure involves Ca2+ spikes in Arabidopsis,Citation10 placing the second Ca2+ spike downstream of NO production and upstream of closure.
These results for ABA-induced and eATP-induced closure inspire several questions. Because these results predominantly concern stomatal closure, an important question is the degree to which ATP-induced opening differs from ATP-induced closure. H2O2 production is required for both ATP-induced opening and ATPγS-induced closure.Citation2,Citation3 If the mechanisms differ, then, they may differ downstream of H2O2, although they may also differ due to amount of H2O2 produced as described above. Interestingly, cytokinin- and auxin-induced stomatal opening involves a decrease in hydrogen peroxide levels,Citation11 as does inhibition of stomatal closure by ethylene in Vicia faba.Citation12 This is in contrast to the requirement for ROS in ATP-induced opening. It should be noted, however, that pretreatment with DTT, a ROS scavenger, prevents light-induced opening (), although the effects of DPI, an NADPH oxidase inhibitor, on light-induced opening have not been tested.
Another important question in plant extracellular nucleotide signaling that needs to be further addressed is possible differences between ADP and ATP signaling. Some results from physiological experiments using ADPβS and ATPγS suggest that eADP and eATP signaling occur through the same receptor, as they have similar effects in similar tissues and are antagonized by the same compounds.Citation2,Citation13–Citation15 However, while eATP induces ROS accumulation in Arabidopsis root cells, eADP does not.Citation16 More significantly, application of ADP induces inward Ca2+ currents within 1 s of application,Citation16 while the first Ca2+ spike does not occur until 30 – 40 s after application of ATP.Citation8 Together, these results suggest that eADP and eATP may act through different receptors. Specifically, the rapid Ca2+ influx caused by ADP in comparison to ATP suggests that ADP may act through an ion channel, while ATP may act through a G protein-linked receptor.
Hao et al. (2012) found that ATP does not induce stomatal opening in gpa1–1 mutants.Citation3 We found that ATPγS does not induce stomatal closing in the gpa1–3 mutants () which indicates that an early step in the signaling pathway for both eATP-induced stomatal opening and closing involves heterotrimeric G proteins. We also found that, in contrast to ATP, ADP is able to induce opening in gpa1–3 mutants (), providing direct evidence that ATP and ADP act through different mechanisms in their regulation of stomatal aperture.
The observation that application of ATPγS and chemical apyrase inhibitors can act synergistically with ABA to induce closure provides additional evidence for cross-talk between extracellular nucleotide and ABA signal pathways during stomatal closure. Taken together these data suggest that the swelling and shrinking of guard cells induced by various stimuli may result in the release of ATP that helps regulate stomatal apertures.
| Abbreviations: | ||
| ABA | = | abscisic acid |
| eATP | = | extracellular ATP |
| ROS | = | reactive oxygen species |
| NO | = | nitric oxide |
| RBOHD/F | = | NADHP oxidase homolog sub-units D/F |
| DTT | = | dithiothreitol |
| DPI | = | diphenyleneiodonium |
| GCA2 | = | growth controlled by ABA 2 |
| ABI2 | = | Abscisic Acid-Insensitive2 |
| PA | = | phosphatidic acid |
| gpa | = | α-subunit of the Arabidopsis heterotrimeric G Protein |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Science Foundation IOS-1027514 to S.J.R. and G.C. and by the Freshman Research Initiative at the University of Texas (funded by Howard Hughes Medical Institute Undergraduate Science Education award 52005907 and National Science Foundation grant CHE–0629136). We thank Alan Jones for providing us gpa1–3 mutant seeds. We also thank Marianna Grenadier for creating the final figures.
References
- Clark G, Roux SJ. Apyrases, extracellular ATP and the regulation of growth. Curr Opin Plant Biol 2011; 14:700 - 6; http://dx.doi.org/10.1016/j.pbi.2011.07.013; PMID: 21855397
- Clark G, Fraley D, Steinebrunner I, Cervantes A, Onyirimba J, Liu A, Torres J, Tang W, Kim J, Roux SJ. Extracellular nucleotides and apyrases regulate stomatal aperture in Arabidopsis. Plant Physiol 2011; 156:1740 - 53; http://dx.doi.org/10.1104/pp.111.174466; PMID: 21636723
- Hao L-H, Wang W-X, Chen C, Wang Y-F, Liu T, Li X, Shang ZL. Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein α subunit and reactive oxygen species. Mol Plant 2012; 5:852 - 64; http://dx.doi.org/10.1093/mp/ssr095; PMID: 22138967
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000; 406:731 - 4; http://dx.doi.org/10.1038/35021067; PMID: 10963598
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 2006; 45:113 - 22; http://dx.doi.org/10.1111/j.1365-313X.2005.02615.x; PMID: 16367958
- Sueldo DJ, Foresi NP, Casalongué CA, Lamattina L, Laxalt AM. Phosphatidic acid formation is required for extracellular ATP-mediated nitric oxide production in suspension-cultured tomato cells. New Phytol 2010; 185:909 - 16; http://dx.doi.org/10.1111/j.1469-8137.2009.03165.x; PMID: 20356346
- Mishra G, Zhang WH, Deng F, Zhao J, Wang XM. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 2006; 312:264 - 6; http://dx.doi.org/10.1126/science.1123769; PMID: 16614222
- Tanaka K, Swanson SJ, Gilroy S, Stacey G. Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol 2010; 154:705 - 19; http://dx.doi.org/10.1104/pp.110.162503; PMID: 20671112
- Murata Y, Pei ZM, Mori IC, Schroeder J. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants (vol 13, pg 2513, 2001). Plant Cell 2002; 14:287
- Dubovskaya LV, Bakakina YS, Kolesneva EV, Sodel DL, McAinsh MR, Hetherington AM, Volotovski ID. cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol 2011; 191:57 - 69; http://dx.doi.org/10.1111/j.1469-8137.2011.03661.x; PMID: 21371039
- Song XG, She XP, He JM, Huang C, Song TS. Cytokinin- and auxin-induced stomatal opening involves a decrease in levels of hydrogen peroxide in guard cells of Vicia faba. Funct Plant Biol 2006; 33:573 - 83; http://dx.doi.org/10.1071/FP05232
- Song XG, She XP, Wang J. Inhibition of darkness-induced stomatal closure by ethylene involves a removal of hydrogen peroxide from guard cells of Vicia faba. Russ J Plant Physiol 2012; 59:372 - 80; http://dx.doi.org/10.1134/S102144371203017X
- Bushart TJ, Cannon AE, Ul Haque A, San Miguel P, Mostajeran K, Clark GB, Porterfield DM, Roux SJ. RNA-seq analysis identifies potential modulators of gravity response in spores of Ceratopteris (Parkeriaceae): evidence for modulation by calcium pumps and apyrase activity. Am J Bot 2013; 100:161 - 74; http://dx.doi.org/10.3732/ajb.1200292
- Clark G, Torres J, Finlayson S, Guan XY, Handley C, Lee J, Kays JE, Chen ZJ, Roux SJ. Apyrase (nucleoside triphosphate-diphosphohydrolase) and extracellular nucleotides regulate cotton fiber elongation in cultured ovules. Plant Physiol 2010; 152:1073 - 83; http://dx.doi.org/10.1104/pp.109.147637; PMID: 20018604
- Clark G, Wu M, Wat N, Onyirimba J, Pham T, Herz N, Ogoti J, Gomez D, Canales AA, Aranda G, et al. Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Mol Biol 2010; 74:423 - 35; http://dx.doi.org/10.1007/s11103-010-9683-7; PMID: 20820881
- Demidchik V, Shang Z, Shin R, Colaço R, Laohavisit A, Shabala S, Davies JM. Receptor-like activity evoked by extracellular ADP in Arabidopsis root epidermal plasma membrane. Plant Physiol 2011; 156:1375 - 85; http://dx.doi.org/10.1104/pp.111.174722; PMID: 21562328