Abstract
Species of the epiphytic fungus Pseudozyma are not pathogenic to plants and can be used as biocontrol agents against plant pathogens. Deciphering how they induce plant defense might contribute to their use for plant protection and expand our understanding of molecular plant–pathogen interactions. Here we show that Pseudozyma aphidis isolate L12, which is known to induce jasmonic acid- and salicylic acid-independent systemic resistance, can also activate local and systemic resistance in an ethylene-independent manner. We also show that P. aphidis localizes exclusively to the surface of the plant leaf and does not penetrate the mesophyll cells of treated leaves. We thus propose that P. aphidis acts via several mechanisms, and is an excellent candidate biocontrol agent.
During their life cycle, plants have to cope not only with harsh and changing environmental conditions, but also with constant attacks by viruses, bacteria, and fungi. To battle these pathogens, growers use various combinations of chemical-based pesticides, insecticides, and fungicides. Environmental concerns and consumer pressure have led to the development of biological control as a viable alternative to the harsh chemicals currently used to control pathogen and insects. Biological control of pathogens can be achieved by one or more mechanisms, including antibiosis, mycoparasitism, competition, and induced resistance in the host plant. The complexity of their mode of action reduces the possibility of resistance buildup in the pathogens.Citation1,Citation2 Induced resistance is of particular importance in the application of biocontrol agents such as Piriformospora indica, Trichoderma asperellum, and Penicillium simplicissimum, which have been shown capable of inducing defense-signaling pathways.Citation2-Citation6 Various signaling molecules have been shown to participate in the induced defense response. The salicylic acid (SA)-mediated defense response, for example, activates the expression of several defense-related genes (e.g., the pathogenesis-related PR1, PR2, and PR5) both locally and systemically. Other plant defense response signaling pathways depend on jasmonic acid (JA) and ethylene (ET).Citation7,Citation8 Among biocontrol agents, epiphytic yeasts are known to act not only by inducing the plant defense response but also as a physical barrier against various plant pathogens by competing with them for space and nutrients.Citation9-Citation17
Of the different types of epiphytic yeasts that colonize plants, those belonging to the genus Pseudozyma (a small group of Basidiomycetes that are related to the UstilaginalesCitation18,Citation19) are of particular interest as biocontrol agents. P. rugulosa and P. flocculosa, for example, have been reported to possess antifungal activity against various powdery mildews.Citation20-Citation26 P. aphidis (isolate CBS517.83), on the other hand, was not associated with novel antifungal activity and biocontrol of powdery mildew.Citation27 However, we recently described the isolation and characterization of a novel P. aphidis strain (isolate L12, Israel 2004) and demonstrated its biocontrol activity against fungal phytopathogens in a dual mode of action which combines antibiosis and induced systemic resistance.Citation28 Isolate L12 was isolated from strawberry leaves, and its identity as a unique P. aphidis strain was confirmed by sequencing the rDNA of the internal transcribed spacer (ITS) and part of the mitochondrial large subunit (mtLSU) and nuclear small subunit (nSSU) genomic regions.Citation28 Structural and growth-pattern studies of isolate L12 revealed that it is a dimorphic epiphytic fungus, i.e., it possesses a yeast-like form and hyphal structures. Microscopic analysis also revealed that P. aphidis isolate L12 can colonize leaf surfaces of both Arabidopsis and tomato plants and that the fungus develops mycelium-like structures on infected tomato leaves. The question still remained whether P. aphidis isolate L12 penetrates the mesophyll cells or is localized exclusively on the surface of plant. To investigate this question, we sprayed Arabidopsis leaves with P. aphidis spores and 3 d later, we fixed the leaves with glutaraldehyde and analyzed the growth pattern and colonization of the fungi on the leaves by scanning electron microscopy, as described by Weigel and Glazbrook.Citation29 shows scanning electron micrographs of the leaves 3 d after application of P. aphidis. While P. aphidis cells were clearly visible on the surface of the treated leaves, they could not be detected inside the mesophyll cells, as reflected by the lack of hyphae/cells inside the plant cells. Moreover, we had shown that tomato and Arabidopsis plants covered with P. aphidis exhibit no pathological symptoms, even 4 weeks post-inoculation with a concentrated inoculum of P. aphidis spores.Citation28 Thus, colonization of the host leaf surface by P. aphidis strain L12 did not seem to negatively influence plant growth or development.
Figure 1. Colonization of Arabidopsis leaf surface by P. aphidis. Scanning electron microscopy of P. aphidis-treated leaf (center panel) demonstrates that while the leaf surface is covered with P. aphidis cells (lower panel), no hyphae are observed inside the mesophyll cells, shown here under a detached trichome (upper panel).

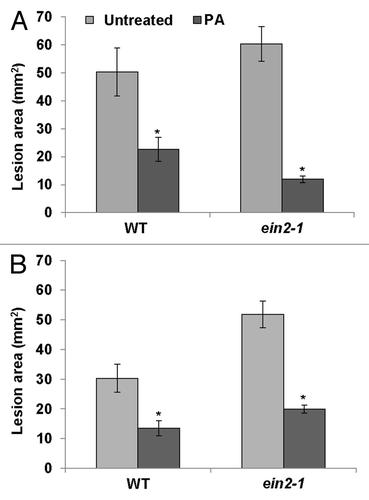
In our previous study, application of P. aphidis on wild-type Arabidopsis plants resulted in induction of not only local, but also systemic resistance to Botrytis cinerea. Furthermore, P. aphidis application was shown capable of inducing systemic resistance in jasmonate resistant 1 (jar1–1), the salicylate hydroxylase-expressing transgenic plant (NahG), and non-expressor of PR1 (npr1–1) Arabidopsis mutants which are impaired in JA signaling and in SA accumulation and signaling, respectively. In light of these observations, we suggested that P. aphidis activates resistance in a JA- and SA-independent manner.Citation28 Even though the P. aphidis-mediated induced resistance was found to be independent of JA and SA signaling, it was well correlated with induction of downstream genes—such as PR1 and PDF1—in these pathways. Moreover, induction of PR1 in P. aphidis-treated plants was antagonized by B. cinerea, when compared with plants that had not been treated with P. aphidis. It is thus possible that in the absence of the pathogen (i.e., B. cinerea), P. aphidis activates the plant defense response by inducing the expression of PR genes that are downstream of both the SA and JA pathways. However, when a necrotrophic pathogen is present, gene expression downstream of the JA response is further enhanced, while the SA-responsive genes are downregulated. We further demonstrated that P. aphidis can lead to recovery of PR1 and PDF1.2 expression in mutants npr1–1 and NahG (but not in jar1–1). Thus, it is possible that the SA/NPR1, but not the JA pathway can be bypassed by P. aphidis to activate PR1 and PDF1.2 expression.Citation28 The resistance activated by P. aphidis in jar1–1 might be due to the activation of other unknown defense-response genes or pathways. Since it has been previously shown that ET signaling is also involved in resistance to B. cinerea,Citation30,Citation31 one pathway that P. aphidis might rely on is the ET-signaling pathway. Here we tested whether P. aphidis can induce local and systemic resistance in Arabidopsis mutant ein2–1 (ethylene-insensitive protein 2), which is impaired in the ET response.Citation30, Citation32 Arabidopsis rosette leaves were treated with P. aphidis and 3 d later, leaves were inoculated with B. cinerea. The disease symptoms caused by B. cinerea were significantly reduced in both wild-type and ein2–1 plants in response to pretreatment with P. aphidis (). We then further tested whether P. aphidis can induce systemic resistance in ein2–1 plants. P. aphidis was applied to one of the Arabidopsis rosette leaves for 24 h, and then untreated leaves were inoculated with B. cinerea. Here, too, we found that P. aphidis can activate the induced systemic response independently of EIN2 signaling (). We thus suggest that P. aphidis activates local and systemic resistance in an ET-independent manner.
Figure 2. Biocontrol of B. cinerea by P. aphidis in Arabidopsis plants. (A) B. cinerea lesion area was measured 72 h after inoculation of the ethylene mutant ethylene-insensitive protein 2 (ein2–1) and wild-type (WT) untreated plants (Untreated) and compared with lesions on their counterparts sprayed with P. aphidis (PA). (B) Lesion area was monitored on systemic leaves of treated (PA) and untreated plants 72 h after infection with B. cinerea. Asterisks denote significant differences (p < 0.05) as determined by Mann-Whitney Rank Sum test.
P. aphidis, a nonpathogenic fungus, holds great promise as a potential biocontrol agent against disease-causing fungal species.Citation28P. aphidis may act via several mechanisms, the most important probably being activation of the plant systemic defense response as it allows the defense signals to propagate to various organs in the developing plant. Interestingly, P. aphidis can bypass not only JA- and SA/NPR1-signaling pathways, but also the ET-signaling pathway to activate their downstream local and systemic responses.
Acknowledgments
This work was supported partially by grant no. IS-4210–09 from the Binational Agricultural Research and Development (BARD) Fund.
Disclosure of Potential Conflicts of Interest
Patent pending: The authors have assigned their rights to Yissum Research Development Company of the Hebrew University of Jerusalem Ltd, which has submitted a patent application entitled “Pseudozyma aphidis as a biocontrol agent against various plant pathogens” (WO 2011/151819 A2). This does not alter our adherence to all Plant Signaling and Behavior policies on sharing data and materials.
References
- Elad Y, Freeman S. Biological control of fungal plant pathogens In: Kempken F, ed. The Mycota, A comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research XI Agricultural Applications. Heidelberg, Germany: Springer 2002: 93-109.
- Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 2010; 48:21 - 43; http://dx.doi.org/10.1146/annurev-phyto-073009-114450; PMID: 20192757
- Stein E, Molitor A, Kogel KH, Waller F. Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol 2008; 49:1747 - 51; http://dx.doi.org/10.1093/pcp/pcn147; PMID: 18842596
- Shoresh M, Yedidia I, Chet I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005; 95:76 - 84; http://dx.doi.org/10.1094/PHYTO-95-0076; PMID: 18943839
- Hossain MM, Sultana F, Kubota M, Koyama H, Hyakumachi M. The plant growth-promoting fungus Penicillium simplicissimum GP17-2 induces resistance in Arabidopsis thaliana by activation of multiple defense signals. Plant Cell Physiol 2007; 48:1724 - 36; http://dx.doi.org/10.1093/pcp/pcm144; PMID: 17956859
- Alfano G, Ivey MLL, Cakir C, Bos JIB, Miller SA, Madden LV, Kamoun S, Hoitink HA. Systemic Modulation of Gene Expression in Tomato by Trichoderma hamatum 382. Phytopathology 2007; 97:429 - 37; http://dx.doi.org/10.1094/PHYTO-97-4-0429; PMID: 18943283
- Heil M, Bostock RM. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann Bot 2002; 89:503 - 12; http://dx.doi.org/10.1093/aob/mcf076; PMID: 12099523
- Koornneef A, Pieterse CM. Cross talk in defense signaling. Plant Physiol 2008; 146:839 - 44; http://dx.doi.org/10.1104/pp.107.112029; PMID: 18316638
- Raacke IC, von Rad U, Mueller MJ, Berger S. Yeast increases resistance in Arabidopsis against Pseudomonas syringae and Botrytis cinerea by salicylic acid-dependent as well as -independent mechanisms. Mol Plant Microbe Interact 2006; 19:1138 - 46; http://dx.doi.org/10.1094/MPMI-19-1138; PMID: 17022178
- Fokkema NJ, van de Laar JAJ, Nellis-Blomberg AL, Schippers B. The buffering capacity of the natural mycoflora of rye leaves to infection by Cochliobolus sativus and its susceptibility to benomyl. Neth J Plant Pathol 1975; 81:176 - 86; http://dx.doi.org/10.1007/BF01976329
- Fokkema NJ, Schippers B. Phyllosphere versus rhizosphere as environments for saprophytic colonization. Cambridge: Cambridge University Press, 1986.
- Avis TJ, Bélanger RR. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa.. Appl Environ Microbiol 2001; 67:956 - 60; http://dx.doi.org/10.1128/AEM.67.2.956-960.2001; PMID: 11157268
- Urquhart EJ, Punja ZK. Hydrolytic enzymes and antifungal compounds produced by Tilletiopsis species, phyllosphere yeasts that are antagonists of powdery mildew fungi. Can J Microbiol 2002; 48:219 - 29; http://dx.doi.org/10.1139/w02-008; PMID: 11989766
- Starmer WT, Ganter PF, Aberdeen V, Lachance MA, Phaff HJ. The ecological role of killer yeasts in natural communities of yeasts. Can J Microbiol 1987; 33:783 - 96; http://dx.doi.org/10.1139/m87-134; PMID: 3690423
- Jacobsen B. Biological Control of Plant Disease by Phyllosphere Applied Biological Control Agents. In: Bailey M, Lilley A, Timms-Wilson T, Spencer-Philips P, eds. Microbial ecology of aerial plant surfaces. London: CABI international, 2006:133-47.
- Bleve G, Grieco F, Cozzi G, Logrieco A, Visconti A. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int J Food Microbiol 2006; 108:204 - 9; http://dx.doi.org/10.1016/j.ijfoodmicro.2005.12.004; PMID: 16443300
- Robiglio A, Sosa MC, Lutz MC, Lopes CA, Sangorrín MP. Yeast biocontrol of fungal spoilage of pears stored at low temperature. Int J Food Microbiol 2011; 147:211 - 6; http://dx.doi.org/10.1016/j.ijfoodmicro.2011.04.007; PMID: 21546110
- Boekhout T. Pseudozyma Bandoni emend. Boekhout, a. genus for yeast-like anamorphs of Ustilaginales. J Gen Appl Microbiol 1995; 41:359 - 66; http://dx.doi.org/10.2323/jgam.41.359
- Avis TJ, Bélanger RR. Mechanisms and means of detection of biocontrol activity of Pseudozyma yeasts against plant-pathogenic fungi. FEMS Yeast Res 2002; 2:5 - 8; PMID: 12702315
- Hajlaoui MR, Traquair JA, Jarvis WR, Bélanger RR. Antifungal activity of extracellular metabolites produced by Sporothrix flocculosa.. Biocontrol Sci Technol 1994; 4:229 - 37; http://dx.doi.org/10.1080/09583159409355331
- Jarvis WR, Shaw LA, Traquair JA. Factors affecting antagonism of cucumber powdery mildew by Stephanoascus flocculosus and S. rugulosus.. Mycol Res 1989; 92:162 - 5; http://dx.doi.org/10.1016/S0953-7562(89)80006-1
- Hajlaou MR, Belanger RR. Antagonism of the yeast-like phylloplane fungus Sporothrix flocculosa against Erysiphe graminis var tritici.. Biocontrol Sci Technol 1993; 3:427 - 34; http://dx.doi.org/10.1080/09583159309355297
- Hajlaou MR, Bélanger RR. Comparative effects of temperature and humidity on the activity of three potential antagonists of rose powdery mildew. Neth J Plant Pathol 1991; 97:203 - 8; http://dx.doi.org/10.1007/BF01989818
- Bélanger RR, Labbé C, Jarvis WR. Commercial-scale control of rose powdery mildew with a fungal antagonist. Plant Dis 1994; 78:420 - 4; http://dx.doi.org/10.1094/PD-78-0420
- Dik AJ, Verhaar MA, Bélanger RR. Comparison of three biological control agents against cucumber powdery mildew (Sphaerotheca fuliginea) in semi-commercial-scale glasshouse trials. Eur J Plant Pathol 1998; 104:413 - 23; http://dx.doi.org/10.1023/A:1008025416672
- Hammami W, Castro CQ, Rémus-Borel W, Labbé C, Bélanger RR. Ecological basis of the interaction between Pseudozyma flocculosa and powdery mildew fungi. Appl Environ Microbiol 2011; 77:926 - 33; http://dx.doi.org/10.1128/AEM.01255-10; PMID: 21115715
- Avis TJ, Caron SJ, Boekhout T, Hamelin RC, Bélanger RR. Molecular and physiological analysis of the Powdery Mildew antagonist Pseudozyma flocculosa and related fungi. Phytopathology 2001; 91:249 - 54; http://dx.doi.org/10.1094/PHYTO.2001.91.3.249; PMID: 18943343
- Buxdorf K, Rahat I, Gafni A, Levy M. The epiphytic fungus Pseudozyma aphidis induces jasmonic acid- and salicylic acid/nonexpressor of PR1-independent local and systemic resistance. Plant Physiol 2013; 161:2014 - 22; http://dx.doi.org/10.1104/pp.112.212969; PMID: 23388119
- Weigel D, Glazebrook J. Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, 2002.
- Thomma BP, Eggermont K, Tierens KF, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea.. Plant Physiol 1999; 121:1093 - 102; http://dx.doi.org/10.1104/pp.121.4.1093; PMID: 10594097
- Díaz J, ten Have A, van Kan JA. The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea.. Plant Physiol 2002; 129:1341 - 51; http://dx.doi.org/10.1104/pp.001453; PMID: 12114587
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 1990; 2:513 - 23; PMID: 2152173