Abstract
Previously, we identified a novel herbivore elicitor-regulated protein in Nicotiana attenuata (NaHER1) that is required to suppress abscisic acid (ABA) catabolism during herbivore attack and activate a full defense response against herbivores. ABA, in addition to its newly defined role in defense activation, mainly controls seed germination and stomatal function of land plants. Here we show that N. attenuata seeds silenced in the expression of NaHER1 by RNA interference (irHER1) accumulated less ABA during germination, and germinated faster on ABA-containing media compared to WT. Curiously, epidermal cells of irHER1 plants were wrinkled, possibly due to the previously demonstrated increase in transpiration of irHER1 plants that may affect turgor and cause wrinkling of the cells. We conclude that NaHER1 is a highly pleiotropic regulator of ABA responses in N. attenuata plants.
Until recently, the plant hormone ABA was mainly thought to regulate a plant’s water regime and its responses to drought, as well as being one of the crucial factors controlling seed germination.Citation1,Citation2 However, the recognition of ABA’s role in plant-pathogen interactions,Citation3 and more recently in plant-insect interactions,Citation4 reveals a larger and highly pleiotropic role played by ABA. This diversity of effects speaks to the importance of hormonal cross talk in plant development, and suggests a role of ABA in mediating tradeoffs between growth and defense.
While searching for genes regulated by specific herbivore-associated elicitors (HAEs) in the ecological model plant, Nicotiana attenuata, we identified NaHER1, a protein of previously unknown function. Upon herbivore attack or during simulated herbivory treatments, the levels of transcripts of this gene were strongly increased,Citation4 suggesting a role in plant defense. From an RNAi-based loss of function analysis of transgenic plants, the NaHER1 protein was found to contribute to ABA homeostatsis by inhibiting the catabolism of the hormone, resulting in increases in leaf ABA levels. From this, we inferred that an increase in ABA was a prerequisite for activating a full defense response in N. attenuata against its herbivores. Second, plants silenced in the expression of NaHER1 were clearly impaired in their responses to ABA, first noticed as reduced growth under drought stress, and under closer examination, as higher transpiration rates during leaf desiccation after ABA treatments.Citation4
Here we explored additional potential roles of NaHER1 in seed germination, as changes in ABA levels and sensitivity are known to be important factors involved in seed germination.Citation2 In addition, other hormones and signaling molecules such as gibberellic acid (GA), jasmonic acid (JA), reactive oxygen species (ROS), nitric oxide (NO), ethylene, cytokinins, and their cross talk, are known to affect significantly seed germination.Citation5-Citation8 The content of endogenous ABA is known to rapidly decline during germination; however, decreases in endogenous ABA are not always associated with increased germination rates.Citation9
In N. attenuata, seed germination is known to be very complex and tightly controlled. It is timed so that seedlings emerge in the immediate post-fire environment,Citation10 providing the germinating seedlings a nutrient-rich environment in which to grow, but also one of intense intra- and inter-specific competition from other plants scrambling to occupy this niche as well as exposure to attack from local herbivore communities that rain in on these vigorously growing plants.Citation11 N. attenuata seeds can stay dormant in soil for up to 150 y between fires, only germinating after being exposed to smoke, which in turn increases endogenous GA and sensitivity to GA,Citation12 decreasing ABA levels, as well as having ABA and 4 additional terpenes that leach from the litter and inhibit germination removed by pyrolysis.Citation13 While elevated endogenous ABA levels in N. attenuata seeds reduce germination,Citation12 as has been shown in other plants, the exact mechanism remains unknown.
Some proteins are known to regulate ABA levels in N. attenuata leaves during water stress, such as the homeodomain-leucine zipper type I protein NaHD20Citation14 and the NaMAPK4 (mitogen-activated protein kinase 4) protein which plays a pivotal role in ABA signaling.Citation15 The NaHER1 gene, which strongly and specifically responds to herbivore cues, such as N-linolenoyl-L-glutamate (C18:3-Glu) contained in oral secretions of Manduca sexta larvae,Citation16 appears to be a novel ABA regulator. Because the function of NaHER1 is to inhibit the catabolism of ABA during herbivory, the ABA pools of irHER1 plants are typically depleted by unfettered catabolism. However, it remained unknown if this ABA deficiency extends to the seeds and germination of irHER1 plants. Following the main work on defense responses,Citation4 additional experiments were conducted to clarify this point.
The biosynthesis of ABA is known to be tissue- and time-specific.Citation17 At first, we found no differences in ABA levels in dry seeds between WT and irHER1 (, 0h). In contrast, ABA levels in irHER1 seeds were significantly lower at 24 and 48 h after sterilizing and placing the seeds on germination media, following standard GA and smoke pre-treatments (). Thus, at the minimum level, the role of NaHER1 indeed extends to seed function, but in an herbivory-independent manner. Since ABA controls many other aspects of plant physiology, e.g., post-germination embryo development, dormancy, stomata opening, water relations, photosynthesis, root geotropism, growth, and adaptation to biotic and abiotic stresses,Citation18 NaHER1 could play additional roles in plant physiology and ecology.
Figure 1. Silencing NaHER1 affected ABA levels in seeds during germination in Nicotiana attenuata. Seeds were sterilized, placed on GB5 media, collected after the designated times, and analyzed by LC-MS/MS for ABA (A). Sterilized seeds were placed on GB5 media supplied with 1µM ABA and germination rate was collected (B). Comparisons were performed by ANOVAs, Fisher’s PLSDs test: ** p ≤ 0.01.
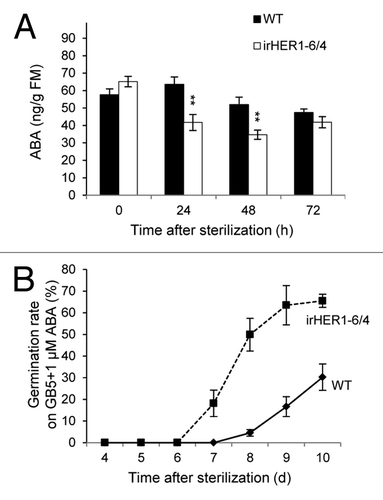
Because ABA levels were reduced in irHER1 seeds (), most likely because NaHER1 suppresses local ABA catabolism in WT seeds, we compared germination rates of irHER1 and WT seeds. Initially, we did not observe any significant changes in germination behavior of irHER1 seedlings under laboratory conditions, which reflected the relatively small changes in ABA content found in the germinating seeds (). Moreover, decreases in endogenous ABA are not always associated with increased germination rates.Citation9 However, when ABA levels are enhanced by exogenously applying 1µM ABA in the standard Gamborg’s B5 media, irHER1 seedlings showed accelerated germination. At 10 d post germination, germination rates of irHER1 seeds were about 2-fold of those found in WT (). These results suggest that NaHER1 gene might affect the ABA levels, and the long-term dormancy of seeds in natural seed banks that is sustained by the terpenes leaching from the litter of dominate vegetation before fires.Citation13 Since N. attenuata is a fire-chasing annual that occupies the post-fire nitrogen-rich soils,Citation10 the tight regulation of germination to time growth in the post fire niche could be influenced and/or regulated by NaHER1.Citation19
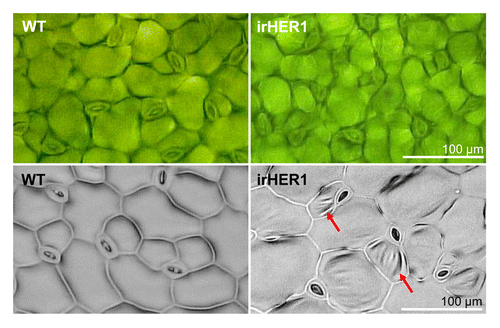
We also carefully inspected cells on leaf surfaces. Surprisingly, epidermal leaf cells in irHER1 plants were wrinkled, as if the cells had lost their turgor and deflated (, upper panels). Although we do not have a direct explanation for this phenotype, it was even more obvious when the leaves were painted with a nail polish and the obtained surface imprints were studied under a light microscope on a flat surface (, lower panels). This phenotype is consistent with the altered transpiration rates and function of NaHER1 in normal plant development under non-stress conditions.Citation4
Figure 2. Leaf surface of wild type and irHER1 plants. Bright field microscopy image (upper row) of leaf surface of WT and irHER1–6/4 plants cultivated in the glasshouse shows wrinkled leaf surface of irHER1 leaf. Surface relief of nail polish strips (lower row) painted and peeled from the leaves after drying and photographed on a flat surface of a microscope slide emphasizes the uneven surface of irHER1 leaf (red arrows).
The regulation of ABA is undoubtedly one of the critical survival strategies in plantsCitation20 that requires homeostatic mechanisms which are environmentally sensitive. Endogenous ABA levels are mainly controlled at the level of biosynthesis and catabolism.Citation21 Oxidation and conjugation are the 2 main pathways to inactivate ABA in which the ABA 8'-hydroxylase and the glucosyltransferases are the 2 key genes.Citation22-Citation24 Although we already demonstrated at least 2 functions of NaHER1 during herbivoryCitation4 and seed germination, how NaHER1 actually regulates ABA catabolism remains unresolved. Ongoing work, including microarray experiments, will assist the future identification of the NaHER1-regulated gene networks and provide additional functional hints for a full understanding of NaHER1 in plant defense and development.
Materials and Methods
Plant material, growth conditions and plant treatments
Seeds of Nicotiana attenuata Torr. ex Watson wild type (WT) and previously established N. attenuata transgenic seeds (irHER1) silenced in the expression of NaHER1Citation4 were germinated on Gamborg’s B5 medium (Duchefa) as described previously.Citation25
ABA analysis
ABA was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on a Varian 1200 Triple-Quadrupole-LC-MS system (Varian, Palo Alto, CA, USA) as described previously.Citation4
Statistical analyses
Analysis of variance (ANOVA) followed by post hoc Fisher's protected least significant difference (PLSD) were used. Comparisons of means were calculated at a minimal 0.05 level of significance.
Microscopy and surface imaging
N. attenuata leaves were freshly removed from the plants and directly observed on a Leica laser microdissection microscope system (Leica, Bensheim, Germany), or the leaf surface was observed indirectly by nail polish method as follows. A thin layer of commercial transparent nail polish was painted on the leaf surface and allowed to dry completely. A small piece of transparent sellotape was placed over each dry nail polish patch, gently peeled together with the attached polish layer, and re-taped on a clean microscope slide. The imprints were observed as above.
Acknowledgments
This work was funded by the Max Planck Society and ERC advanced grant, ClockworkGreen (No. 293926) to ITB. Son Truong Dinh was supported by the Vietnam Ministry of Agricultural and Rural Development and Deutscher Akademischer Austauschdienst (DAAD) and the Max Planck Society. We thank Dr John T Christeller for critically reading the manuscript and for his helpful suggestions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 1998; 282:287 - 90; http://dx.doi.org/10.1126/science.282.5387.287; PMID: 9765153
- Nambara E, Okamoto M, Tatematsu K, Yano R, Seo M, Kamiya Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci Res 2010; 20:55 - 67; http://dx.doi.org/10.1017/S0960258510000012
- Fan J, Hill L, Crooks C, Doerner P, Lamb C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol 2009; 150:1750 - 61; http://dx.doi.org/10.1104/pp.109.137943; PMID: 19571312
- Dinh ST, Baldwin IT, Galis I. The HERBIVORE ELICITOR-REGULATED1 gene enhances abscisic acid levels and defenses against herbivores in Nicotiana attenuata plants. Plant Physiol 2013; 162:2106 - 24; http://dx.doi.org/10.1104/pp.113.221150; PMID: 23784463
- Sarath G, Hou G, Baird LM, Mitchell RB. ABA, ROS and NO are key players during switchgrass seed germination. Plant Signal Behav 2007; 2:492 - 3; http://dx.doi.org/10.4161/psb.2.6.4575; PMID: 19704595
- Ranjan R, Lewak S. Jasmonic acid promotes germination and lipase activity in non-stratified apple embryos. Physiol Plant 1992; 86:335 - 9; http://dx.doi.org/10.1034/j.1399-3054.1992.860222.x
- Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 2000; 122:415 - 24; http://dx.doi.org/10.1104/pp.122.2.415; PMID: 10677434
- Koornneef M, Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) heynh. Theor Appl Genet 1980; 58:257 - 63; http://dx.doi.org/10.1007/BF00265176
- Braun JW, Khan AA. Endogenous abscisic Acid levels in germinating and nongerminating lettuce seed. Plant Physiol 1975; 56:731 - 3; http://dx.doi.org/10.1104/pp.56.6.731; PMID: 16659382
- Baldwin IT, Morse L. Up in smoke: II. Germination of Nicotiana attenuata in response to smoke-derived cues and nutrients in burned and unburned soils. J Chem Ecol 1994; 20:2373 - 91; http://dx.doi.org/10.1007/BF02033208
- Preston CA, Baldwin IT. Positive and negative signals regulate germination in the post-fire annual, Nicotiana attenuata.. Ecology 1999; 80:481 - 94
- Schwachtje J, Baldwin IT. Smoke exposure alters endogenous gibberellin and abscisic acid pools and gibberellin sensitivity while eliciting germination in the post-fire annual, Nicotiana attenuata.. Seed Sci Res 2004; 14:51 - 60; http://dx.doi.org/10.1079/SSR2003154
- Krock B, Schmidt S, Hertweck C, Baldwin IT. Vegetation-derived abscisic acid and four terpenes enforce dormancy in seeds of the post-fire annual, Nicotiana attenuata.. Seed Sci Res 2002; 12:239 - 52; http://dx.doi.org/10.1079/SSR2002117
- Ré DA, Dezar CA, Chan RL, Baldwin IT, Bonaventure G. Nicotiana attenuata NaHD20 plays a role in leaf ABA accumulation during water stress, benzylacetone emission from flowers, and the timing of bolting and flower transitions. J Exp Bot 2011; 62:155 - 66; http://dx.doi.org/10.1093/jxb/erq252; PMID: 20713465
- Hettenhausen C, Baldwin IT, Wu J. Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol 2012; 158:759 - 76; http://dx.doi.org/10.1104/pp.111.190074; PMID: 22147519
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 2001; 125:711 - 7; http://dx.doi.org/10.1104/pp.125.2.711; PMID: 11161028
- Koiwai H, Nakaminami K, Seo M, Mitsuhashi W, Toyomasu T, Koshiba T. Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol 2004; 134:1697 - 707; http://dx.doi.org/10.1104/pp.103.036970; PMID: 15064376
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 1988; 39:439 - 73; http://dx.doi.org/10.1146/annurev.pp.39.060188.002255
- Kermode AR. Role of abscisic acid in seed dormancy. J Plant Growth Regul 2005; 24:319 - 44; http://dx.doi.org/10.1007/s00344-005-0110-2
- Farnsworth E. The ecology and physiology of viviparous and recalcitrant seeds. Annu Rev Ecol Syst 2000; 31:107 - 38; http://dx.doi.org/10.1146/annurev.ecolsys.31.1.107
- Cutler AJ, Krochko JE. Formation and breakdown of ABA. Trends Plant Sci 1999; 4:472 - 8; http://dx.doi.org/10.1016/S1360-1385(99)01497-1; PMID: 10562731
- Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 2005; 56:165 - 85; http://dx.doi.org/10.1146/annurev.arplant.56.032604.144046; PMID: 15862093
- Oritani T, Kiyota H. Biosynthesis and metabolism of abscisic acid and related compounds. Nat Prod Rep 2003; 20:414 - 25; http://dx.doi.org/10.1039/b109859b; PMID: 12964836
- Xu ZJ, Nakajima M, Suzuki Y, Yamaguchi I. Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol 2002; 129:1285 - 95; http://dx.doi.org/10.1104/pp.001784; PMID: 12114582
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 2002; 12:177 - 83; http://dx.doi.org/10.1007/PL00012666