Abstract
Pitcher plants of the genus Nepenthes capture a wide range of arthropod prey for nutritional benefit, using complex combinations of visual and olfactory signals and gravity-driven pitfall trapping mechanisms. In many localities throughout Southeast Asia, several Nepenthes different species occur in mixed populations. Often, the species present at any given location have strongly divergent trap structures and preliminary surveys indicate that different species trap different combinations of arthropod prey, even when growing at the same locality. On this basis, it has been proposed that co-existing Nepenthes species may be engaged in niche segregation with regards to arthropod prey, avoiding direct competition with congeners by deploying traps that have modifications that enable them to target specific prey types. We examined prey capture among 3 multi-species Nepenthes populations in Borneo, finding that co-existing Nepenthes species do capture different combinations of prey, but that significant interspecific variations in arthropod prey combinations can often be detected only at sub-ordinal taxonomic ranks. In all lowland Nepenthes species examined, the dominant prey taxon is Formicidae, but montane Nepenthes trap few (or no) ants and 2 of the 3 species studied have evolved to target alternative sources of nutrition, such as tree shrew feces. Using similarity and null model analyses, we detected evidence for niche segregation with regards to formicid prey among 5 lowland, sympatric Nepenthes species in Sarawak. However, we were unable to determine whether these results provide support for the niche segregation hypothesis, or whether they simply reflect unquantified variation in heterogeneous habitats and/or ant communities in the study sites. These findings are used to propose improvements to the design of field experiments that seek to test hypotheses about targeted prey capture patterns in Nepenthes.
Introduction
The carnivorous syndrome is thought to have evolved independently in plants on at least 6 occasions.Citation1,Citation2 The 14 plant families that are currently recognized as containing carnivorous species belong to 5 Orders and comprise more than 650 species. Diversity in the structure and complexity of carnivorous plant traps, as well as methods of operation, is considerable. In addition to the well-known, spring-trap mechanism of the Venus’ Flytrap (Dionaea muscipula (Droseraceae)), carnivorous plants deploy traps with adhesive surfaces (e.g., Drosera (Droseraceae), Pinguicula (Lentibulariaceae) and Byblis (Byblidaceae)), gravity-operated slippery pitfalls (e.g., Sarracenia (Sarraceniaceae), Nepenthes (Nepenthaceae) and Cephalotus (Cephalotaceae)), and suction or eel-type traps (Utricularia, Genlisea (Lentibulariaceae)).Citation1
This diversity of trapping mechanisms among carnivorous plant families has intrigued scientists for over a century, and in recent decades the rate of publication of studies that seek to explain the evolution, diversification, and mode(s) of operation of carnivorous plant traps has increased dramatically.Citation3-Citation11 A current question of interest is whether any species display prey trapping behavior that is consistent with the evolution of suites of characteristics that facilitate the “targeted” capture of specific prey taxa, or are they simply passive sampling traps that capture different prey taxa in proportion to their abundances in the surrounding habitat?
Recent research into this question has focused on the pitcher plant genera, Sarracenia and Nepenthes, as they display considerable intra-generic diversification and modification to trap structure ().Citation12 In several Nepenthes species, targeted prey capture strategies have been detected and documented. These specialized nutrient sequestration strategies are facilitated by unique morphological features and/or extreme modifications to trap structure. For instance, pitchers of Nepenthes albomarginata have a band of dense trichomes around the orifice which are fed upon by termites of the genus Hospitalitermes (Blattodea: Termitoidae) which, in turn, comprise the majority of this species’ prey;Citation13,Citation14 Nepenthes ampullaria derives a significant amount of N from leaf-litter by deploying a “carpet” of modified pitchers, whose geometry facilitates the capture of falling debris;Citation15 while Nepenthes rajah supplements the N derived from trapping arthropods by also “capturing” tree shrew feces with its outsized, highly modified pitchers.Citation16 The detection of these extraordinary nutrient acquisition strategies raises the possibility that less conspicuous, but equally specialized strategies could occur in Nepenthes species that trap only arthropods.Citation12 If found to be widespread in Nepenthes, specialized prey capture strategies could be recognized as a key evolutionary driver of speciation in the genus. This, coupled with the fact that the genus has a broad, but patchy geographical distribution throughout the Malay Archipelago, means that Nepenthes has potential to serve as a model system to investigate how combinations of ecological, environmental, and geographical processes have influenced the evolution of highly specialized plant organs and their associated interactions with animals.
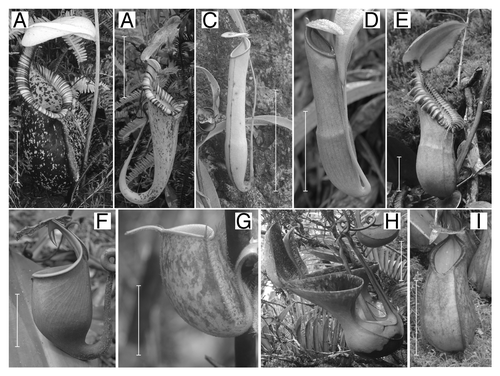
Figure 1. Interspecific variation in Nepenthes pitcher structure, as demonstrated by the Nepenthes species examined in this study. (A) N. rafflesiana, lower pitcher; (B) N. rafflesiana, upper pitcher; (C) N. gracilis, (D) N. mirabilis, (E) N. macrophylla, upper pitcher; (F) N. bicalcarata, upper pitcher; (G) N. ampullaria pitcher, (H) N. lowii, upper pitcher; (I) N. tentaculata, lower pitcher. Scale bar = 5 cm.
As carnivorous plants are unable to physically pursue their prey, and the production of traps incurs metabolic costs, potential prey must be attracted to the traps (and then caught by them) in sufficient numbers for the carnivorous syndrome to be of net benefit to the plant.Citation17-Citation19 Accordingly, carnivorous plants in general, and pitcher plants in particular, deploy combinations of visual and olfactory signals (as well as nutritious rewards to visitors in the form of nectar), to attract arthropods to them.Citation20 Different arthropod taxa respond to different combinations of cues, so a Nepenthes species that targets a specific prey type can be expected to produce pitchers with combinations of characteristics that are tuned to the sensitivity maxima of that prey.
At present, relatively little is known about how attractive cues operate in pitcher plants. Research in this fieldCitation20,Citation21 lags behind studies of trap function,Citation3,Citation5,Citation16,Citation22 due to the simple fact that the mechanical aspects of trap operation are easier to observe and manipulate in controlled field experiments. Another impediment to progress in this area is that in order to determine how (or if) specialized combinations of plant-produced signals target a particular prey type, it is necessary to establish first that the prey is actually being targeted. Once more, research in this area is still in its infancy. Studies of specialized nutrient acquisition strategies in Nepenthes have focused on the most conspicuous, easily detected examples, such as the capture of mammal feces,Citation5,Citation7 or “unusual” arthropod prey, such as termites.Citation14 For Nepenthes species that lack unique pitcher characteristics and trap only arthropods, little is known about whether or not they target any particular types of prey. This presents a barrier to further research on the development and role of attraction cues and trapping mechanisms, as it is difficult to effectively test hypotheses relating to targeted prey capture syndromes until firm evidence of targeted capture is obtained. Furthermore, the structure, diversity, and composition of the arthropod communities in the habitats in which Nepenthes grow is virtually unknown, making it difficult to determine whether or not differences in the proportions of various arthropod taxa trapped by Nepenthes indicate the existence of a targeted capture strategy, or merely reflect the relative abundances and diversity of arthropod taxa in the surrounding habitat.
Existing evidence for specialization
Most Nepenthes species trap only arthropods.Citation23,Citation24 The first detailed investigation into prey capture patterns in a single Nepenthes speciesCitation20 examined the contents of 262 pitchers of Nepenthes rafflesiana in Brunei (). Identified prey belonged to 3 arthropod classes and 15 Orders. The dominant prey taxon was Formicidae, which accounted for 64.3–88.7% of all prey caught. Other prominent Orders included Diptera, Blattodea (Termitoidae), Coleoptera, Thysanoptera, non-formicid Hymenoptera and Lepidoptera. In addition, dimorphism in N. rafflesiana pitchers plays an important role in the type and number of different prey taxa that are caught.Citation20 So-called “lower pitchers,” which are ovoid and produced by the plant at ground level, trapped less volant prey than “upper pitchers,” which are funnel shaped, produced on climbing stems and may be positioned several meters above the ground. The upper pitchers emit a fragrance which attracts volant prey, whereas the lower pitchers do not. Thus, within a single Nepenthes species, differences in pitcher characteristics appear to facilitate alternative prey-capture strategies, enabling the plant to exploit volant prey where it is most abundant in the habitat – above the ground, at the canopy level of the vegetation in which the plants grow.
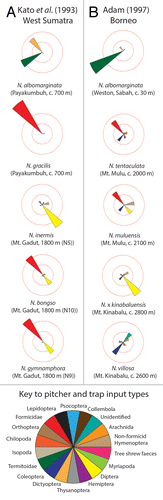
The greatest diversity of Nepenthes species occurs on the islands of Borneo and Sumatra, both of which support more than 30 species.Citation25,Citation26 In many habitats that are suitable for Nepenthes on these islands, 2 to 4 (and sometimes up to 8) species may occur at a single site. In such cases, the diversity of trap morphologies among these species is often high. In a study of prey capture among 10 species of Nepenthes within a small geographical area in West Sumatra,Citation27 arthropod prey from 14 Orders was detected, as was evidence for targeted prey capture strategies in at least 2 species (). Formicidae was the numerically dominant prey taxon in pitchers of 8 of the Nepenthes species examined, but N. albomarginata trapped large numbers of termites, whereas Nepenthes inermis caught mostly Diptera. Qualitative field observations of prey capture by N. inermis indicate that the small, yellow pitchers of this species are particularly attractive to Diptera, and this species appears to target them as its primary source of arthropod prey.Citation26,Citation27
Figure 2. Prey capture patterns in Nepenthes species studied by (A) Kato et al.Citation23 and (B) Adam.Citation24 Each prey taxon is represented as a “wedge” in the chart. The size of each wedge is scaled according to the proportion of prey that belonged to that taxon. The scale is represented by concentric circles: the inner one denotes 25% of prey, whereas the outer one denotes 50% of prey.
In a survey of prey captured by 18 Nepenthes taxa in western Borneo, a total of 15 arthropod Orders was detected.Citation28 Once more, Formicidae was the numerically dominant prey taxon in most species, but some taxa from very high altitudes did not capture any ants (). Comparisons of similarity in prey composition using Sorensen’s Coefficient revealed that different Nepenthes species appear to trap different combinations of prey taxa. Although this result could be anticipated (given the widely divergent habitat types and geographical locations that were included in this studyCitation28), the lowest estimates of similarity were obtained for groups of lowland Nepenthes that grew at the same locations, indicating that species that occur in mixed, multi-species populations might exploit different combinations of arthropod prey taxa.
Thus, from an ecological perspective, Nepenthes in Borneo and Sumatra is characterized by high levels of species richness, frequent occurrence in mixed, multi-species populations, numerical dominance of prey by Formicidae (in most species), plus a few species that have highly specialized, atypical N sequestration strategies. This combination of characteristics has prompted scientists to broaden the scope of their investigations to search for evidence of more subtle levels of specialization among species that trap only arthropods. Given that unique morphological characteristics or visual cues facilitate specialized arthropod capture strategies,Citation5,Citation14,Citation21,Citation22 could less obvious or less extreme variations to trap structure could also be involved in targeted capture of particular arthropod taxa? If so, what ecological processes might drive subtle evolutionary divergence in trap structure? It has been proposed that Nepenthes could be a candidate model for adaptive radiation, using modifications to trap geometry to target a wide range of N sources, particularly in habitats where Formicidae is rare.Citation16 Alternatively, it has been suggested that the diversity of trap morphologies that is often observed in mixed, multi-species populations of Nepenthes could be evidence for prey partitioning or niche segregation.Citation29 This hypothesis predicts that sympatric Nepenthes species may avoid direct competition for arthropod prey by exploiting different components of the local arthropod fauna.
Although the comparative prey analyses from Nepenthes in SumatraCitation27 and BorneoCitation28 seem to indicate that some Nepenthes species specialize on different arthropod prey (and in the case of N. albomarginata, this evidence is strongCitation14), their analyses did not directly address the question about whether any of these species are truly specialists. Ellison and Gotelli examined patterns of prey capture among different carnivorous plant Families, testing a series of hypotheses about specialization and niche segregation.Citation2 First, they examined whether or not various carnivorous plant genera differ in their levels of specialization toward prey. Comparative analysis (using ANOVA) of estimates of Hurlbert’sCitation30 index of the probability of an interspecific encounter (PIE) among prey revealed significant differences, indicating that some carnivorous plant genera appear to be more specialized than others. Next, they posed the question, “Are [any carnivorous plant genera] really specialists?” By analyzing estimates (and associated confidence limits) of similarity in prey capture using Chao’sCitation31 modified version of the Jaccard’s Index (Jchao), they found no evidence that the prey captured by carnivorous plants differs from that caught by passive, artificial traps placed in the plants’ habitats. This result indicates that, at the genus level, the traps of carnivorous plants function in a similar manner to simple passive traps of similar size and shape. On this basis, it was assumed that (at the genus level) visual or olfactory attractants produced by the traps of carnivorous plants do not contribute significantly to the taxonomic composition of their prey, and that in the case of passive trapping mechanisms, “the selectivity of a trap can be understood largely based on the simple geometry of its size, shape, and orientation.” However, this result raises an obvious question: given that species such as Nepenthes aristolochioides have been shown to use visual signals to attract dipteran prey,Citation21 why then, do they invest resources in the production of these cues if they confer no significant benefits?
Finally, Ellison and GotelliCitation2 examined prey caught by mixed, multi-species populations of Drosera and Sarracenia at 11 different geographical locations, to see whether there was any evidence of interspecific, intrageneric niche segregation. Null-model analysis of measures of Pianka’sCitation32 index of overlap in resource useCitation33 was used to test the hypothesis that observed patterns of prey capture among sympatric carnivorous plant species were significantly more extreme than those that would occur by chance. In all cases, high degrees of niche overlap (rather than niche segregation) were detected, indicating that mixed, multi-species populations of carnivorous plants do not avoid competition by exploiting different arthropod prey taxa. On this basis, Ellison and GotelliCitation2 concluded that competition for limiting resources does not drive diversification in sympatric carnivorous plant species from the same genus.
For the most part, Ellison and GotelliCitation2 conducted their analyses on carnivorous plant genera, rather than species, so the hypotheses they tested have yet to be applied to prey capture patterns in individual Nepenthes species. However, their methods are suitable for this purpose and could be useful in detecting specialized arthropod prey capture strategies, thereby establishing a basis for further research into the mechanisms by which carnivorous plants signal their potential prey. In this study, we investigated prey capture patterns in 8 Nepenthes species at 3 localities in Borneo. Four of these species are already known to have specialized N sequestration strategies. Our primary objective was to determine whether evidence for specialized prey capture strategies could be detected using sampling and analytical methods that have been used in previous studies.Citation20,Citation27,Citation28 We also tested for evidence of niche segregation in mixed, multi-species populations of Nepenthes. On the basis of our findings, we review the effectiveness of these methodologies and propose improvements for future research.
Results
Prey capture patterns
In common with findings of previous studies,Citation20,Citation24,Citation27,Citation28 the pitchers that we surveyed trapped a wide range of arthropod prey (; ), including 12 Orders and 17 formicid taxa. Formicidae was the numerically dominant taxon in the pitchers of all 5 lowland species surveyed, but N. bicalcarata and N. ampullaria also trapped large numbers of termites (; ). By contrast, Diptera was the numerically dominant arthropod prey taxon in pitchers at Trusmadi, with no termites and only 2 individuals of Formicidae being trapped at this site (one by a pitcher of N. lowii, another by a pitcher of N. macrophylla) (; ). Overall, pitchers on Mount Trusmadi captured 11 arthropod Orders, as did pitchers at the lowland sites, indicating that the diversity of arthropod Orders trapped by Nepenthes is comparable in both the lowlands and highlands. However, at the level of the individual pitcher, lowland pitchers trapped significantly more prey items (means: lowland pitchers = 32.78 ± 8.63; highland pitchers = 2.31 ± 0.31; Kruskall-Wallace Test, H1 = 52.49, P < 0.001) and prey taxa (means: lowland pitchers = 3.02 ± 0.17; highland pitchers = 1.71 ± 0.17; Kruskall-Wallace Test, H1 = 21.56, P < 0.001) than highland ones. Comparisons of both numbers of prey items and numbers of prey Orders among all 9 Nepenthes species demonstrated that although significant interspecific differences exist for both of these variables (Kruskall-Wallace Tests: for prey numbers: H7 = 40.14, P < 0.001; for numbers of prey Orders: H9 = 68.20, P < 0.001), post-hoc comparisons revealed that the majority of species pairs that were significantly different involved N. lowii, which trapped almost no arthropod prey (). These results support the hypothesis that N. lowii upper pitchers have virtually lost the ability to trap arthropods,Citation5 but also show that there is little interspecific variation in these statistics among other Nepenthes.
Figure 3. Prey capture patterns (with prey resolved to Order) in Nepenthes pitchers and artificial traps at (A) Serian, (B) Sandakan, and (C) Trusmadi.
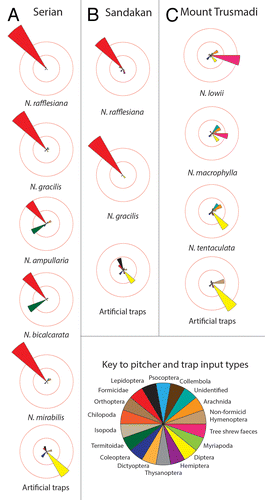
Table 1. Mean prey numbers (± 1 SE) per pitcher according to different arthropod orders caught by the different Nepenthes species at each study site
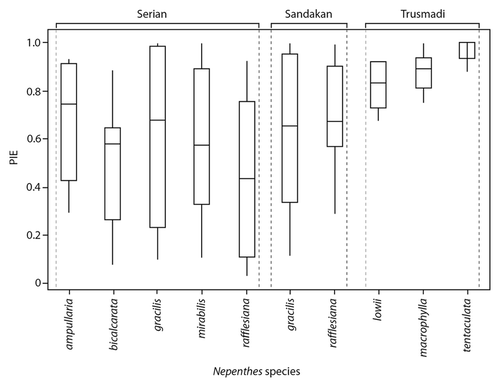
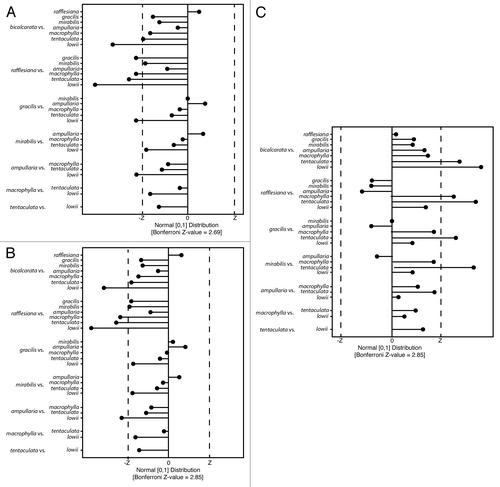
Figure 4. Results of post-hoc pairwise analysis of prey caught by each Nepenthes species, using Dunn’s Tests. In each graph, any points that lie outside the interval defined by -Z – Z denote significant differences. (A) Numbers of prey items trapped; (B) Numbers of prey taxa trapped; (C) Values for the Probability of an Interspecific Encounter (PIE).
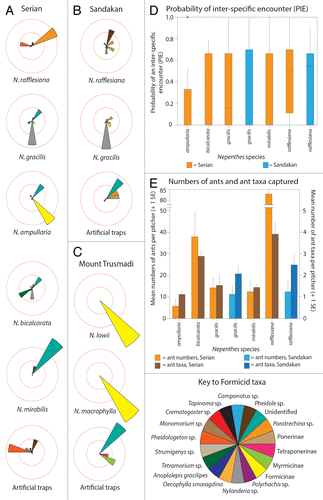
Comparative analysis of formicid prey capture revealed that lowland Nepenthes trapped a wide range of taxa, but montane Nepenthes caught almost none (). Several of the lowland Nepenthes species appeared to trap different combinations of formicid taxa (), a pattern that was supported in part by subsequent similarity and null-model analyses (see below). The only ant-capture pattern that was common to both Sandakan and Serian was for Nepenthes gracilis, whose pitchers trapped large numbers of Nylanderia sp. (Formicinae) workers. Given that these sites are 950 km apart, this finding might not be coincidental and the hypothesis that N. gracilis has evolved to target these ants as a major source of N is worthy of further investigation.Citation22
Figure 5. Formicid prey capture by Nepenthes and artificial traps at (A) Serian, (B) Sandakan, and (C) Trusmadi. (D) Results of analysis of Probability of an Interspecific Encounter (PIE) for formicid prey at Serian and Sandakan. For each variable, boxes illustrate the median (horizontal line), upper, and lower quartiles (limits of the box), and upper and lower deciles (limits of the vertical lines). (E) Mean numbers (± 1 SE) of formicid prey items and taxa found in pitchers at Serian and Sandakan.
Arthropod capture by artificial traps
There were no significant differences in numbers of arthropod individuals or Orders captured by artificial traps at lowland vs. highland sites (Mann-Whitney tests for prey numbers, U = 94.0, P > 0.05; for prey Orders: U = 82.5, P > 0.05). These results contradict those for pitchers and indicate that the density and diversity of arthropods in the plants’ habitats are unaffected by altitude.
Artificial traps captured significantly greater numbers and diversity of arthropods than Nepenthes pitchers (, and ; Mann-Whitney tests for prey numbers, U = 3175.5, P < 0.01; for prey taxa, U = 3735.0, P < 0.01). These results support the hypothesis that Nepenthes pitchers capture only a “subset” of the local arthropod fauna, rather than passively sampling from all taxa present in the surrounding habitat.
Table 2. Summary of arthropod capture by artificial traps at each study site. All values are means ± 1 SE
Do Nepenthes species differ in terms of specialization on arthropod prey?
Analysis of prey capture spectra using PIE, with prey identified to Ordinal rank, indicates that there are significant inter-specific differences in levels of specialization on arthropod prey (; Kruskall-Wallace Test, H9 = 42.71, P < 0.001). However, post-hoc, pairwise analysis of medians using Dunn’s tests demonstrated that the only significantly different species pairs were between N. tentaculata and 4 of the lowland species (N. bicalcarata, N. gracilis, N. mirabilis, and N. rafflesiana) (). Thus, N. tentaculata appears to be comparatively unspecialized, whereas the other species have similar levels of specialization toward prey at the Ordinal rank (i.e., known specialists such as N. ampullaria, N. bicalcarata, N. lowii, and N. macrophylla have similar values of PIE to N. mirabilis, N. gracilis, and N. rafflesiana). There were no significant differences in PIE among lowland Nepenthes species for formicid prey (; Kruskall-Wallace Test, H6 = 42.71, P = 0.937). This result does not indicate that these Nepenthes species are un/specialized with regards to Formicidae; rather, it demonstrates that there are no significant inter-specific differences in levels of specialization, even though some species (e.g., N. bicalcarata and N. rafflesiana) trap more ants and ant taxa than their congeners ().
Do different Nepenthes species specialize on particular prey?
Comparative analysis of prey capture by Nepenthes species and artificial traps (using estimates of Jchao) showed that at the Ordinal level, the composition of prey caught by N. gracilis, N. mirabilis, N. rafflesiana, and N. tentaculata pitchers has very high levels of similarity to that caught by artificial traps, suggesting that these species do not target any particular arthropod Orders (). However, the prey caught by the other 4 species, N. ampullaria, N. bicalcarata, N. lowii, and N. macrophylla, were significantly different to those caught by artificial traps, reinforcing the conclusions of previous authors that these Nepenthes species do specialize in trapping certain types of “prey.” For N. lowii and N. macrophylla, this result is not surprising, as their specialized resource-exchange mutualisms with tree shrews are well-documented. This result also provides support for the argument that the methods used in this study can detect highly atypical, specialized N sequestration strategies in Nepenthes. However, the results for N. ampullaria and N. bicalcarata are less easily interpreted. Both of these species are known to have modified, highly specialized N sequestration strategies,Citation15,Citation34,Citation35 but the effects of these could not be measured in this study. Rather, the differences in similarity that we detected reflect the fact that N. ampullaria and N. bicalcarata pitchers trapped substantial numbers of termites, whereas the artificial traps did not. The foraging behavior of termites is highly specific,Citation36 and the probability of termites visiting artificial traps that lack any apparent attractants to these insects is low. To date, the only Nepenthes species that has been demonstrated to target termites is N. albomarginata,Citation14 but we have found them in pitchers of N. ampullaria and N. bicalcarata pitchers on several occasions, so the possibility that these Nepenthes are somehow predisposed to trapping termites cannot be excluded. This could be due simply to their position in the habitat (unlike most other Nepenthes species, these species grow under the forest canopy, where termites are abundant), as much as any modifications to pitcher characteristics.
Figure 7. Results of the similarity analysis for Nepenthes pitchers at Serian, Sandakan, and Trusmadi. The plotted values are Chao–Jaccard abundance-based similarity index JChao, adjusted for unobserved taxa (Chao et al., 2005); The horizontal lines through each point denote 95% parametric confidence intervals that were derived from 10000 bootstrap samples. (A) All prey taxa, with all ants treated as a single taxon (Formicidae), (B) Formicid taxa only.
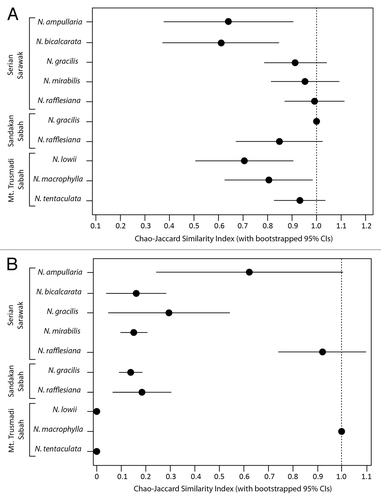
In terms of formicid prey capture, the only species that showed no significant differences in similarity to the artificial traps were N. ampullaria and N. rafflesiana (the latter at Serian only)( and ). This result indicates that N. bicalcarata, N. gracilis, N. mirabilis, and N. rafflesiana (at Sandakan) are not passive sampling traps, but that they trap different combinations of formicid taxa to artificial traps. By contrast, N. ampullaria traps relatively few ants and does not seem to target any particular taxa, whereas N. rafflesiana traps more ants than any other type of prey,Citation20 and appears to attract a very wide range of formicid taxa.
Evidence for niche segregation among mixed, multi-species populations of Nepenthes
At the Ordinal level, no evidence of niche segregation was detected among mixed populations of Nepenthes at any of the sites examined (). However, when formicid prey only were analyzed, evidence for significant niche segregation was detected at both Serian and Sandakan (). Examination of the raw data from Serian indicated that N. rafflesiana and N. gracilis were the species that were most clearly segregated from the others (). These 2 species, which are generally found in open, disturbed sites, also demonstrated very strong segregation from N. mirabilis, which colonizes similar habitats. By contrast, the formicid prey of N. ampullaria, N. bicalcarata, and N. mirabilis showed considerably greater overlap.
Table 3. Summary of null model analysis of niche overlap in prey utilization. Observed is the observed average pairwise niche overlap. Expected is the mean value of average pairwise niche overlap in 10000 randomizations of the resource utilization data. The P value is the upper tail probability of finding the observed pattern if the data were drawn from the null distribution
Discussion
Our findings reflect those of previous studiesCitation20,Citation27,Citation28 and demonstrate that Nepenthes pitchers trap a wide range of arthropod prey types. Although the numerically dominant prey taxon in the lowland sites was Formicidae, ants were very scarce in pitchers at high altitudes on Mount Trusmadi. The highly specialized N sequestration strategies of N. lowii and N. macrophylla were detected through analysis of similarity, but we used surrogate estimates of tree shrew faecal capture, which could have over- or under-estimated the actual rate of “capture” of this resource. Despite this potential source of error, our results indicate that the sampling and analytical methods chosen can detect gross differences in nutrient sequestration strategies in Nepenthes and that in terms of arthropod prey capture, significant differences in the composition of arthropod prey do exist among different Nepenthes species. The patterns of prey capture that we found in the lowland Nepenthes are also similar to those returned by earlier studies, indicating that the methodologies we used yielded data of similar quality, even though some previous analyses used larger sample sizesCitation20 or longer observational periods.Citation27,Citation28 On this basis, we conclude that our data are representative of typical prey-capture patterns in Nepenthes (insofar as these are presently known) and are therefore suitable for testing hypotheses about targeted prey capture and niche segregation. However, the veracity of our findings was limited by a number of technical and logistical constraints, several of which only became apparent after the data were analyzed. We consider the detection of these constraints to be of value in advancing knowledge in this field. Accordingly, the discussion that follows focuses mainly upon these factors, and how they can be addressed in future research into prey capture patterns in Nepenthes.
Limitations to the experimental design
The comparatively short observation period (14 d) that we used could have resulted in the lower numbers and diversity of prey captured by pitchers surveyed, compared with previous studies,Citation27,Citation28 which did not account for the amount of time pitchers had been able to trap prey prior to sampling. However, as a key objective of our study was to be able to identify formicid prey to the lowest possible taxonomic rank, we limited the duration of our experiments in order to prevent the metazoan pitcher fauna from breaking up the prey and making it more difficult to identify them accurately. We contend that this protocolCitation20 is superior as all traps operated for the same period of time, thereby permitting us to compare prey input rates and providing data that were better suited to testing for evidence for niche segregation at sub-ordinal taxonomic ranks.
The artificial traps were considerably larger than Nepenthes pitchers, fewer in number, and could not provide a truly representative estimate of the diversity and relative abundance of all arthropod taxa at each of the study sites. Rather, they provided a surrogate estimate, trapping those arthropods that entered them by chance (i.e., the unbiased sampling scheme that we sought to implement was successful), or were attracted to them by visual and/or olfactory cues that we were either unaware of, or could not eliminate (i.e., uncontrollable sources of error or bias affected the data). This approach meant that it was not possible to directly compare prey capture rates between artificial traps and pitchers. Despite this shortcoming, the artificial traps caught different assemblages of arthropods to Nepenthes pitchers ( and ), demonstrating that collectively, Nepenthes do not passively “sample” prey at random from the surrounding habitat.
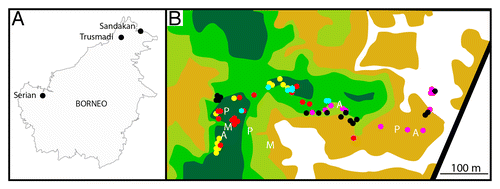
At Serian, the heterogeneous vegetation and aggregated distributions of the 5 Nepenthes species present meant that only one or 2 Nepenthes species co-occurred at any given location within the study area (). Thus, only 5 of a possible 10 pairs of co-occurring Nepenthes species combinations actually occurred at this site. On the basis of our unquantified field observations, this pattern appears to be common at locations were several species of Nepenthes occur within a small geographical area. Rather than growing in a single, mixed-species population comprising all of the Nepenthes species present at a site, groups of 2 or 3 species tend to occupy different niches within the site, and may be spatially isolated from other groups by sharply defined ecotones (). It is not yet known whether high levels of habitat heterogeneity affect the structure and composition of arthropod communities within small geographical areas like Serian, but they are unlikely to be uniform.Citation37
Figure 8. Study sites. (A) Map of Borneo, showing the locations of the 3 study sites. (B) Map of the “Serian” site, showing vegetation types and boundaries, and locations of pitchers sampled. Key to symbols: Locations of pitchers: N. ampullaria – blue dots, N. bicalcarata – yellow dots, N. gracilis – black dots, N. mirabilis – pink dots, N. rafflesiana – red dots. Locations of artificial traps: A – arboreal pitfall, P – terrestrial pitfall, M – malaise. Vegetation types: white – open, bare ground with sparse grasses and shrubs, beige – open, with sparse shrubs and dense grass, light green – highly degraded peat swamp forest, with dense, low shrubs, green – secondary peat swamp forest, with remnant canopy and some gaps, dark green – closed, intact peat swamp forest. The black line represents a sealed road. Scale bar = 100 min.
Interspecific variation in prey capture patterns in Nepenthes
Regardless of any shortcomings in the experimental design, significant interspecific variation in the diversity and composition of prey trapped by Nepenthes have been detected in all multi-species surveys published to date, demonstrating that Nepenthes that grow in mixed, multi-species populations do not all trap the same types of prey in the same numbers. The numerical dominance by Formicidae in pitchers of all lowland species suggests that this prey taxon is worthy of more detailed study, particularly at the species level. The significant differences in composition of formicid prey among Nepenthes species within a very small geographical area at Serian (; ), provides qualified support for the hypothesis that Nepenthes pitchers are not passive sampling traps, and that sympatric Nepenthes avoid competition by targeting different Formicid prey taxa.
Ants are an insignificant component of prey in pitchers on Mount Trusmadi. However, artificial traps placed in the same habitat trapped ants, indicating that Formicidae is present at the site. The scarcity of ant prey in pitchers could reflect either a low density of ants in upper montane habitats, or an inability of Nepenthes to effectively attract and/or trap them in large numbers. The density and diversity of terrestrial Formicidae at approximately 2000 min asl on Mount Mulu (240 km SW of Mount Trusmadi) is significantly lower than at nearby sites at low altitudes,Citation38 but we have observed a few species of ants to be relatively common on the upper slopes of this mountain.Citation5 Furthermore, N. tentaculata pitchers sampled at 1800 min asl on Mount Mulu trapped substantial numbers of ants.Citation28 On tropical mountains, the relationship between altitude and formicid density and diversity remains poorly understood, but it is clear from our findings, as well as those from other studies,Citation27,Citation28 that for whatever reason, a considerable proportion of montane Nepenthes species examined to date trap few ants. Thus, even if ants are not particularly rare on high tropical mountains, they do not comprise a significant source of nutrients for Nepenthes. This might place selective pressure on Nepenthes to exploit alternative sources of supplementary N.Citation5
Recent research into the structure and function of the pitcher components responsible for trapping and retaining prey (i.e., the peristome, the waxy zone, and viscoelastic fluids),Citation39-Citation42 suggests that Nepenthes species in montane, perhumid climates, are more likely to have pitchers with broad peristomes, highly viscoelastic fluids and reduced (or no) waxy zones. This is because the peristome is highly effective at trapping prey when it is wet, but is much less effective when it is dry (Bohn and Federle).Citation3 As most high mountains in Borneo rarely experience any sort of water deficit, broad peristomes are likely to be effective almost all of the time. Reduced waxy zones and viscoelastic fluids are also thought to be more effective at trapping flying insects rather than crawling ones (such as ants), whereas the reduced waxy zone/highly viscoelastic fluid combination seems to be more effective at trapping flying prey.Citation39 However, none of the 3 montane species in our study fit these generalized patterns. All have watery pitcher fluid, 2 have well-developed waxy zones (N. macrophylla and N. tentaculata), while 2 have narrow peristomes (N. lowii and N. tentaculata). Yet, none of them trapped significant numbers of ants, and Diptera accounted for a greater proportion of prey than in any of the lowland species at Serian or Sandakan. While the general trend toward broad peristomes, viscoelastic pitcher fluids and reduced waxy zones in perhumid montane habitats currently has broad support among scientists, there may be instances where high degrees of specialization toward particular environments, or N sequestration strategies, overrides this pattern. One such example is Nepenthes campanulata, which grows on sheer limestone cliffs in lowland habitats on Borneo and Palawan.Citation43 This lowland species produces bell-shaped pitchers that have a well-developed waxy zone, watery pitcher fluid and a highly reduced peristome. This combination of characteristics would normally indicate specialization toward non-volant prey, such as ants. However, most of its prey are volant, with Diptera accounting for almost 60% of identifiable prey.Citation43 Not only does N. campanulata trap a large proportion of volant prey with a highly developed waxy zone, it appears that its unique pitcher structure is primarily a function of the highly specialized environment that it inhabits.Citation43
Based on analysis of PIE, there appears to be little inter-specific variation in levels of specialization toward prey by Nepenthes. This result is intriguing, as the highly specialized N sequestration strategies of N. lowii and N. macrophylla were readily detected by similarity analysis of Jchao, even when prey was only identified to Ordinal rank. Taken together, these results imply that species that trap only arthropods, such as N. gracilis, N. mirabilis and N. rafflesiana, have similar levels of specialization to N. lowii and N. macrophylla. Formicidae is the dominant prey taxon in each of these species, so if they possess specialized prey capture strategies these could target particular, distinct combinations of ant species ( and ). Alternatively, it is feasible that there are significant differences in levels of specialization toward arthropod prey among the Nepenthes species studied, but as most prey taxa were not resolved below ordinal rank, these could not be detected through analysis of PIE.
Within the same, relatively homogeneous habitat (e.g., within each site at Sandakan and Trusmadi), different Nepenthes species caught different combinations of prey, with results of analysis of similarity and null model analysis providing qualified support for the niche segregation hypothesis (, ). By contrast, although some of the species at Serian also caught different combinations of prey (particularly Formicidae), the vegetation at this site is highly heterogeneous, so there are alternative, competing interpretations for this result, which are discussed below.
Are lowland Nepenthes species engaged in niche segregation with regards to formicid prey?
The fact that we detected evidence of niche segregation among lowland Nepenthes species toward formicid prey is noteworthy in 2 respects. First, our results lend support to the argument of Ellison and Gotelli,Citation2 that identifying prey only to Ordinal rank is likely to obscure prey capture strategies that target specific arthropod genera or species. Second, the question as to whether our results provide direct support for the niche segregation hypothesis remains a matter of conjecture. At Serian, the results of null model analysis for formicid prey provided strong support for the niche segregation hypothesis (). However, at Sandakan, where 2 Nepenthes species grow together in the same vegetation type (i.e., the potential effects of habitat heterogeneity are neutralized), the calculated P value is close to the threshold of 0.05 (). It is therefore possible that the high degree of habitat heterogeneity at Serian is a confounding factor in the design of this experiment. For instance, Nepenthes that grow in different vegetation types could be passively trapping prey from different (potentially mutually exclusive) formicid communities, rather than targeting specific prey from a “common pool” of potential prey species, all of which are equally available to all Nepenthes at the site. Furthermore, the structure and composition of the ant community at Serian has the potential to be heterogeneous with regards to both vegetation types (and levels of disturbance) as well as vertical position within a given vegetation type.Citation37,Citation44,Citation45 Despite the fact that the results of similarity analysis indicate that some Nepenthes are specialists, we cannot determine whether the divergent formicid prey capture patterns we detected among the 5 Nepenthes species at Serian are due to niche segregation or simple passive capture of different formicid species from different niches within a highly heterogeneous site. It is feasible even that niche segregation could exist within the ant community rather than the Nepenthes, so that the prey capture patterns merely reflect the spatial positioning pitchers in both the vegetation and the (partitioned) ant community.
Are we using the right statistics—does numerical dominance of prey by one taxon reflect the nutritional value of that taxon to the plant?
Previous studies have sought to detect targeted prey capture in Nepenthes by comparing the abundances of different prey taxa. As a consequence, there has been a tendency to view numerical dominance by a particular prey taxon as evidence that this prey is being targeted by the plant species in question.Citation2,Citation27,Citation28 However, the nutritional value of prey to Nepenthes is likely to vary, both among arthropod taxa and in relation to the rate at which any given taxon is trapped. It is possible that numerical dominance by a given prey type does not equate to nutritional “dominance.” For instance, the capture of one large blattid could be of greater nutritional value to a plant than the capture of many small ants. This raises the possibility that some Nepenthes species have evolved to target prey taxa that represent a small proportion of the total catch, but which account for a high proportion of assimilated nutrients. A candidate example of this strategy is Nepenthes muluensis, which occurs on a few high mountains in northern Sarawak. In a study of the contents of N. muluensis pitchers on Mount Mulu, 36.5% of prey items were Formicidae, while workers of the Asian honeybee, Apis cerana (Hymenoptera: Apiidae) accounted for 23%.Citation28 We have observed A. cerana workers to be trapped by N. muluensis upper pitchers on a regular basis on Mount Mulu and it is possible that this species has evolved to target them as prey. These insects are many times larger than workers of all formicid species that we have observed on the upper slopes of Mount Mulu. Although the nutritional value of these 2 N sources to N. muluensis is not yet known, it is feasible that this species could “target” A. cerana through a specialized combination of visual and/or olfactory signals,Citation25 whereas Formicidae is not targeted, but nevertheless represents a valuable “bycatch.” This hypothesis currently lacks empirical support, but it illustrates the possibility that targeted prey capture in Nepenthes has the potential to be more complex than has previously been assumed, particularly with regards to the numbers of each prey taxon caught. We therefore contend that in order to detect targeted prey capture patterns in Nepenthes, it is preferable to determine the nutritional value of each prey taxon to the plant a priori, and to use numerical dominance as an indicator of targeted prey capture strategies only if this is found to have a strong, positive relationship to high levels of assimilated nutrients in the plants’ tissues.
Summary
Nepenthes growing in mixed, multi-species populations catch different combinations of arthropod prey and on the basis of comparative analysis of formicid prey capture patterns, could be engaged in niche segregation. If so, this might be facilitated by divergent attractive cues and or capture mechanisms among sympatric Nepenthes species that serve to target different prey taxa. In species that trap only arthropods, targeted prey capture strategies are likely to operate below the taxonomic rank of Order, but in order to determine whether any particular arthropod taxon is being targeted, it is necessary to establish that it is of significantly greater nutritional value to the plant than other prey.
Rather than providing strong empirical support for the targeted prey capture or niche segregation hypotheses, the findings of this study serve to bring to light many of the key variables that have yet to be considered (let alone incorporated) into field experiments. Furthermore, they remind us that despite the great improvements in our understanding of the biology of Nepenthes that have been made over the last 2 decades, this progress has been uneven. We still have a great deal to learn about the relationships between these plants and their environment, and the structure of the arthropod communities they exploit. In all but the most aberrant species, we require at least some of this information before we can elucidate the mechanisms by which the plants signal and capture their prey.
Materials and Methods
Study sites
The study was performed at 3 geographically isolated sites in Malaysian Borneo (). The sites were chosen on the basis of several criteria: accessibility and suitability for running extended field experiments, inter-site variation in altitude, at least 2 species of Nepenthes occurring at each site, variation in habitat heterogeneity in at least one site and, where possible, a mixture of Nepenthes species, including some that are already known to have specialized N sequestration strategies, along with others that have yet to be studied in this respect. The first site (called “Serian” in this study), was in Sarawak, near the town of Serian, SE of Kuching, at an altitude of 37 min above sea level (asl). Precise details of the location of this site are sensitive and are not presented here, but can be provided to bona fide researchers if necessary. This site supports fragments of several types of vegetation, ranging from open, bare sandy ground to intact peat swamp forest (). Originally, the area supported a mosaic of peat swamp forest and tropical heath forest (kerangas), much of which has now been cleared. Five species of Nepenthes occur at this site: N. ampullaria, N. bicalcarata, N. gracilis, N. mirabilis, and N. rafflesiana. Two species (N. ampullaria and N. bicalcarata) are virtually confined to the intact peat swamp forest fragments, whereas N. rafflesiana occurs around the edges of the same fragments (both under and outside the forest canopy), and N. gracilis and N. mirabilis are abundant in open, exposed areas. provides an outline of the vegetation types and boundaries at this site, as well as the locations of Nepenthes pitchers that were sampled. As the Nepenthes species at this site do not all grow in the same vegetation type, it is possible that their pitchers were exposed to different arthropod faunas.
The second site (referred to as “Sandakan”), was at Sandakan Rainforest Park, approximately 5.5 km W of the town of Sandakan in Sabah (). This site is administered by the Sabah Forestry Department and Sandakan Municipal Council. Two species of Nepenthes, N. gracilis, and N. rafflesiana, grow in mixed populations on an open, exposed embankment (altitude: 29 min asl) adjacent to a nearby racecourse. The vegetation is sparse adinandra belukar, which is characterized by patches of bare ground, interspersed with patches of resam fern (Dicranopteris lineariz) and small shrubs.Citation46 This site was chosen because it is geographically disjunct from Serian, but has a similar altitude and climate. Furthermore, N. gracilis and N. rafflesiana are also found at Serian, thereby permitting a comparisons of prey capture patterns in 2 similar environments that are geographically disjunct. However, in contrast to N. rafflesiana and N. gracilis at Serian, these species grow together in mixed populations the same habitat at Sandakan, and are exposed to the same arthropod fauna.
The third site (called “Trusmadi”) was on the summit ridge of Mount Trusmadi, in central Sabah (). Three species of Nepenthes (N. lowii, N. macrophylla, and Nepenthes tentaculata) occur in montane “mossy forest” on the upper slopes of this mountain, from altitudes ranging from 2000–2642 min asl. Pitchers were sampled along the summit trail in an altitudinal range of 2200–2500 min asl and were produced by plants that grew in mossy forest by the trail. Although the vegetation appeared to be homogeneous throughout the altitudinal range of the study site, it is possible that the arthropod fauna varied along the altitudinal gradient. This site was chosen because of its high altitude and the fact that N. lowii and N. macrophylla are known to have specialized N acquisition strategies: both species have evolved to trap tree shrew feces.Citation5,Citation16 The upper pitchers of Nepenthes lowii are thought to have effectively lost the ability to trap arthropod prey,Citation5 but those of N. macrophylla appear to be less specialized and trap a variety of arthropods.Citation16 On tropical mountains, arthropod density is thought to decrease as altitude increases,Citation38,Citation47 and it has been suggested that this could place selective pressure on Nepenthes to exploit alternative N sources.Citation5,Citation16 Accordingly, we chose this site to determine whether the specialized N sequestration strategies of N. lowii and N. macrophylla could be detected using the analytical methods used by Ellison and Gotelli,Citation2 and to compare patterns of arthropod prey capture in montane Nepenthes with those from the lowlands. The third species present at this site, N. tentaculata, lacks any obvious specializations toward prey capture, and is thought to trap only arthropods.Citation25,Citation28
Sampling methods
Pitchers were selected for study on the basis of their age and condition. Very old and very young pitchers, or those that displayed obvious signs of damage, may not be fully functional and hence were excluded. Pitchers that could only be accessed by leaving established trails and damaging fragile vegetation on Mt Trusmadi were also excluded. Maximum sample sizes were imposed by logistical constraints. We had sufficient human resources to study 10–25 pitchers of each species at each site. The final numbers sampled were the number of pitchers that were still intact and operational at the end of the experiment (). All pitchers sampled were from separate plants. Where pronounced intra-specific pitcher dimorphism occurred,Citation20 we attempted to sample equal numbers of both pitcher types, to account for potential effects of dimorphism (however, although N. lowii exhibits extreme levels of dimorphism, only the large, toilet-shaped upper pitchers produced by mature plants were studied (), due to a scarcity of lower pitchers). If more than 25 suitable pitchers of a given species could be found at a site, 20–25 of these were randomly selected for study. If less than 20 suitable pitchers were available, all of them were tagged and used.
We used the method of Moran to survey arthropod prey capture in Nepenthes pitchers.Citation20 This involves clearing the pitchers of their existing contents and “re-setting” them, so that they capture prey for a fixed, uniform period, thereby enabling direct comparison of capture rates among all pitchers (Note: other comparative studies of prey capture in NepenthesCitation27,Citation28 did not measure the precise ages of the pitchers they examined, or periods time over which their prey was captured. Inter-specific variation in pitcher longevity among different Nepenthes species is known to be significant,Citation18,Citation25 and could have pronounced effects on prey numbers and diversity). Pitchers were emptied of their contents and rinsed with distilled water. The contents were then passed through filter paper to remove all macroscopic detritus. The volume of the fluid was then measured to the nearest ml and returned to the pitcher, whereas the detritus was discarded. Pitchers were then left for 14 d to capture prey. This time interval was chosen as it allows the longest possible period for pitchers to trap prey without providing the invertebrate fauna sufficient time to re-colonize the pitchers and degrade the prey to the point where identification becomes difficult.Citation48 At the conclusion of the experiment, the contents of the pitchers were poured into a 250 ml beaker and the inner surfaces of the pitchers were thoroughly rinsed with distilled water to remove all of the contents. The fluid was filtered once more, but this time the contents were retained and preserved in 70% ethanol for sorting and identification, whereas the filtrate was returned to the pitchers.
Remains of captured prey were sorted and identified to Order where possible.Citation49-Citation51 To investigate prey capture patterns below the rank of Order, all Formicidae were then identified to the lowest possible taxonomic rank. In accordance with previous findings,Citation5,Citation16 pitchers of N. lowii and N. macrophylla both trapped tree shrew feces, but as the scats disintegrate upon contact with the pitcher fluid, they could not be counted accurately. In order to incorporate faecal input rates into our quantitative analyses, we used a surrogate estimate of capture, based on observed capture rates from a different study.Citation52 We scored any species that definitely received faecal inputs during the study period as “positive,” and for each of these pitchers, assumed a rate of 3 instances of defecation by tree shrews into pitchers every 14 d.
Surveys of local arthropod fauna using artificial traps
Artificial traps were deployed to determine the range of arthropod prey available to Nepenthes at each site. No attempt was made to match the morphological characteristics of the artificial traps to those of Nepenthes pitchers as this was not an objective of the study. Three types of traps were used: terrestrial pitfall traps, arboreal pitfall traps, and malaise traps. All traps were unbaited and passive. Ethylene glycol was used as the killing agent for the malaise traps, but alcohol was utilized for the pitfall traps because ethylene glycol is toxic and easily accessible to small mammals when used in pitfall traps. The traps were operated for 7 days. At each site, 3 terrestrial pitfalls, 3 arboreal pitfalls and 2 malaise traps were set. Traps were positioned among the Nepenthes plants, in order to maximize the chances of obtaining a passive sample of the arthropod fauna that is exploited by the plants.
Data analysis
All summary statistics are presented as means ± 1 SD. As no data sets met the assumptions of parametric analytical methods and no suitable transformations were found, non-parametric methods were used to compare measures of location, using Minitab v.16. All decisions about hypotheses were made against a critical value of α = 0.05.
Quantitative analysis of prey capture patterns were conducted the Ordinal level for all main arthropod taxa (except Formicidae, which was treated as a separate taxon due to its importance in lowland pitchers), and at the sub-Ordinal rank for taxa within Formicidae.
, , and comprise “star plots,” which display proportional abundances of prey taxa for each Nepenthes species, in which each taxon is represented by a “wedge” in a circular chart.Citation2 The size of the wedge was scaled in proportion to the amount of total prey that was accounted for by any given taxon. All taxa listed in the key were trapped by pitchers; taxa that are not visible in any of the star plots were either not captured at all, or were not captured in sufficient numbers to be resolved. The minimum level of resolution was in the star plots was 4 percent of total prey caught.
To determine whether different Nepenthes species specialize on particular prey taxa, we compared estimates of Hurlburt’s PIE,Citation30 using a Kruskall-Wallace test and Dunn’s post-hoc tests. To compare captured prey to available prey (i.e., that caught by artificial traps), we calculated values of the Chao-Jaccard Index (JChao) using the EstimateS software package.Citation53 2000 bootstrap replications were used to estimate the parametric 95% confidence intervals for the point-estimates of JChao. To test for evidence of niche segregation, we performed null-model analysis using the ‘RA-3′ algoritjm within the EcoSim software package to quantify niche overlap using Pianka’sCitation32 index of overlap in resource use.Citation2,Citation54
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Lee Ying Fah, Richard Majapun, Jarry Lajanga, Harry Abbie, and other staff of Sabah Forestry Department. Heon Sui Peng, Chou Lee Yiung, Leong Shen Nyan, Lee Yoon Ling, and Lee Sin Henn assisted in the fieldwork. Monash University provided financial and logistical support. Sabah Forestry Department provided technical assistance for the sorting and identification of arthropods.
References
- Juniper BE, Robins RJ, Joel D. The Carnivorous Plants. London, UK: Academic Press, 1989.
- Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants--Darwin’s ‘most wonderful plants in the world’. J Exp Bot 2009; 60:19 - 42; http://dx.doi.org/10.1093/jxb/ern179; PMID: 19213724
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc Natl Acad Sci U S A 2004; 101:14138 - 43; http://dx.doi.org/10.1073/pnas.0405885101; PMID: 15383667
- Bauer U, Willmes C, Federle W. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Ann Bot 2009; 103:1219 - 26; http://dx.doi.org/10.1093/aob/mcp065; PMID: 19307189
- Clarke CM, Bauer U, Lee CC, Tuen AA, Rembold K, Moran JA. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biol Lett 2009; 5:632 - 5; http://dx.doi.org/10.1098/rsbl.2009.0311; PMID: 19515656
- Gaume L, Perret P, Gorb E, Gorb S, Labat JJ, Rowe N. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arthropod Struct Dev 2004; 33:103 - 11; http://dx.doi.org/10.1016/j.asd.2003.11.005; PMID: 18089026
- Grafe TU, Schöner CR, Kerth G, Junaidi A, Schöner MG. A novel resource-service mutualism between bats and pitcher plants. Biol Lett 2011; 7:436 - 9; http://dx.doi.org/10.1098/rsbl.2010.1141; PMID: 21270023
- Adamec L. Mineral nutrition of carnivorous plants: a review. Bot Rev 1997; 63:273 - 99; http://dx.doi.org/10.1007/BF02857953
- Thum M. Segregation of habitat and prey into two sympatric carnivorous plant species, Drosera rotundifolia and Drosera intermedia.. Oecologia 1986; 70:601 - 5; http://dx.doi.org/10.1007/BF00379912
- Rivadavia F, Kondo K, Kato M, Hasebe M. Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA Sequences. Am J Bot 2003; 90:123 - 30; http://dx.doi.org/10.3732/ajb.90.1.123; PMID: 21659087
- Müller KF, Borsch T, Legendre L, Porembski S, Barthlott W. Recent progress in understanding the evolution of carnivorous lentibulariaceae (lamiales). Plant Biol (Stuttg) 2006; 8:748 - 57; http://dx.doi.org/10.1055/s-2006-924706; PMID: 17203430
- Bauer U, Clemente CJ, Renner T, Federle W. Form follows function: morphological diversification and alternative trapping strategies in carnivorous Nepenthes pitcher plants. J Evol Biol 2012; 25:90 - 102; http://dx.doi.org/10.1111/j.1420-9101.2011.02406.x; PMID: 22023155
- Moran JA, Merbach MA, Livingston NJ, Clarke CM, Booth WE. Termite prey specialization in the pitcher plant Nepenthes albomarginata – evidence from stable isotope analysis. Ann Bot (Lond) 2001; 88:307 - 11; http://dx.doi.org/10.1006/anbo.2001.1460
- Merbach MA, Merbach DJ, Maschwitz U, Booth WE, Fiala B, Zizka G. Mass march of termites into the deadly trap. Nature 2002; 415:36 - 7; http://dx.doi.org/10.1038/415036a; PMID: 11780106
- Moran JA, Clarke CM, Hawkins BJ. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. Int J Plant Sci 2003; 164:635 - 9; http://dx.doi.org/10.1086/375422
- Chin L, Moran JA, Clarke C. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytol 2010; 186:461 - 70; http://dx.doi.org/10.1111/j.1469-8137.2009.03166.x; PMID: 20100203
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist nutrient-poor habitats. Am Nat 1984; 124:479 - 97; http://dx.doi.org/10.1086/284289
- Osunkoya OO, Daud SD, Wimmer FL. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Ann Bot 2008; 102:845 - 53; http://dx.doi.org/10.1093/aob/mcn162; PMID: 18757449
- Karagatzides JD, Ellison AM. Construction costs, payback times, and the leaf economics of carnivorous plants. Am J Bot 2009; 96:1612 - 9; http://dx.doi.org/10.3732/ajb.0900054; PMID: 21622347
- Moran JA. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. J Ecol 1996; 84:515 - 25; http://dx.doi.org/10.2307/2261474
- Moran JA, Clarke C, Gowen BE. The use of light in prey capture by the tropical pitcher plant Nepenthes aristolochioides. Plant Signal Behav 2012; 7:957 - 60; http://dx.doi.org/10.4161/psb.20912; PMID: 22836498
- Bauer U, Di Giusto B, Skepper J, Grafe TU, Federle W. With a flick of the lid: a novel trapping mechanism in Nepenthes gracilis pitcher plants. PLoS One 2012; 7:e38951; http://dx.doi.org/10.1371/journal.pone.0038951; PMID: 22719998
- Joel D. Mimicry and mutualism in carnivorous pitcher plants (Sarraceniaceae, Nepenthaceae, Cephalotaceae, Bromeliaceae). Biol J Linn Soc Lond 1988; 35:185 - 97; http://dx.doi.org/10.1111/j.1095-8312.1988.tb00465.x
- Jebb MHP. An account of Nepenthes in New Guinea. Sci New Guinea 1991; 17:7 - 54
- Clarke C. Nepenthes of Borneo. Kota Kinabalu, Malaysia: Natural History Publications, 1997.
- Clarke C. Nepenthes of Sumatra and Peninsular Malaysia. Kota Kinabalu: Malaysia, Natural History Publications, 2001.
- Kato M, Hotta M, Tamin R, Itino T. Inter- and intra-specific variation in prey assemblages and inhabitant communities in Nepenthes pitchers in Sumatra. Trop Zool 1993; 6:11 - 25; http://dx.doi.org/10.1080/03946975.1993.10539206
- Adam JH. Prey spectra of Bornean Nepenthes species (Nepenthaceae) in relation to their habitat. Pertanika Journal of Tropical Forest Science 1997; 20:121 - 34
- Bauer U, Grafe TU, Federle W. Evidence for alternative trapping strategies in two forms of the pitcher plant, Nepenthes rafflesiana. J Exp Bot 2011; 62:3683 - 92; http://dx.doi.org/10.1093/jxb/err082; PMID: 21459766
- Hurlbert SH. The non-concept of species diversity: a critique and alternative parameters. Ecology 1971; 52:577 - 56; http://dx.doi.org/10.2307/1934145
- Chao A, Chazdon RL, Colwell RK, Shen TJ. A new statistical approach for assessing compositional similarity based on incidence and abundance data. Ecol Lett 2005; 8:148 - 59; http://dx.doi.org/10.1111/j.1461-0248.2004.00707.x
- Pianka ER. The structure of lizard communities. Annu Rev Ecol Syst 1973; 4:53 - 74; http://dx.doi.org/10.1146/annurev.es.04.110173.000413
- Gotelli NJ, Graves GR. Null models in ecology. Washington, DC: Smithsonian Institution Press, 1996.
- Clarke CM, Kitching RL. Swimming ants and pitcher plants: a unique ant-plant interaction from Borneo. J Trop Ecol 1995; 11:589 - 602; http://dx.doi.org/10.1017/S0266467400009160
- Bonhomme V, Gounand I, Alaux C, Jousselin E, Barthelemy D, Gaume L. The plant-ant Camponotus schmitzi helps its carnivorous host-plant Nepenthes bicalcarata to catch its prey. J Trop Ecol 2010; 27:1 - 10; http://dx.doi.org/10.1017/S0266467410000532
- Jones DT, Gathorne-Hardy F. Foraging activity of the processional termite Hospitalitermes hospitalis (Termitidae: Nautitermitinae) in the rain forest of Brunei, north-west Borneo. Insectes Soc 1995; 42:359 - 69; http://dx.doi.org/10.1007/BF01242164
- Dejean A, Corbara B. A review of mosaics of the ants (Hymenoptera:Formicidae) and associates on Clerodendrum (Verbenaceae) in Australia. Aust J Entomol 2003; 42:109 - 13; http://dx.doi.org/10.1046/j.1440-6055.2003.00339.x
- Collins NM. The distribution of soil macrofauna on the west ridge of Gunung (Mount) Mulu, Sarawak. Oecologia 1980; 44:263 - 75; http://dx.doi.org/10.1007/BF00572689
- Bonhomme V, Pelloux-Prayer H, Jousselin E, Forterre Y, Labat JJ, Gaume L. Slippery or sticky? Functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytol 2011; 191:545 - 54; http://dx.doi.org/10.1111/j.1469-8137.2011.03696.x; PMID: 21434933
- Bauer U, Clemente CJ, Renner T, Federle W. Form follows function: morphological diversification and alternative trapping strategies in carnivorous Nepenthes pitcher plants. J Evol Biol 2012; 25:90 - 102; http://dx.doi.org/10.1111/j.1420-9101.2011.02406.x; PMID: 22023155
- Benz MJ, Gorb EV, Gorb SN. Diversity of the slippery zone microstructure in pitchers of nine carnivorous Nepenthes taxa. Arthropod-Plant Interact 2012; 6:147 - 58; http://dx.doi.org/10.1007/s11829-011-9171-2
- Moran JA, Gray LK, Clarke C, Chin L. Capture mechanism in Palaeotropical pitcher plants (Nepenthaceae) is constrained by climate. Ann Bot 2013; 112:1279 - 91; http://dx.doi.org/10.1093/aob/mct195; PMID: 23975653
- Clarke C, Lee CC. Observations of the natural history and ecology of Nepenthes campanulata.. Carnivorous Plant Newsletter 2014; 43 In press
- Bluthgen N, Stork NE, Fiedler K. Bottom-up control and co-occurrence in complex communities: honeydew and nectar determine a rainforest ant mosaic. Oikos 2004; 106:344 - 58; http://dx.doi.org/10.1111/j.0030-1299.2004.12687.x
- Basset Y, Novotny Y, Kitching RL, Miller SE. Arthropods of forests: dynamics and resource use tropical spatio-temporal dynamics and resource use in the canopy. Cambridge, United Kingdom: Cambridge University Press, 2003.
- Sim JWS, Tan HTW, Turner IM. Adinandra belukar: an anthropogenic heath forest in Singapore. Vegetatio 1992; 102:125 - 37; http://dx.doi.org/10.1007/BF00044729
- Samson DA, Rickart EA, Gonzales PC. Ant diversity and abundance along an altitudinal gradient in the Philippines. Biotropica 1997; 29:349 - 63; http://dx.doi.org/10.1111/j.1744-7429.1997.tb00436.x
- Beaver RA. Biological studies of the fauna of pitcher plants (Nepenthes) in West Malaysia. [Nouvelle Serie] Ann Soc Entomol Fr 1979; 15:3 - 17
- Chung AYC. Common lowland rainforest ants of Sabah. Kota Kinabalu, Malaysia: Natural History Publications, 1995.
- Mohammad M. Keys to the terrestrial invertebrates. Kota Kinabalu, Malaysia: Universiti Malaysia Sabah, 1999.
- Hill D, Abang F. The insects of Borneo. Kota Samarahan, Malaysia: Universiti Malaysia Sarawak, 2005.
- Greenwood M, Clarke C, Lee CC, Gunsalam A, Clarke RH. A unique resource mutualism between the giant Bornean pitcher plant, Nepenthes rajah, and members of a small mammal community. PLoS One 2011; 6:e21114; http://dx.doi.org/10.1371/journal.pone.0021114; PMID: 21695073
- Colwell RK. EstimateS: Statistical estimation of species richness and shared species from samples, version 7.5. 2005; http://purl.oclc.org/estimates
- Gotelli NJ, Entsminger GL. 2007. EcoSim: null models software for ecology, version 7. Jericho, Vermont: Acquired Intelligence Inc. & Kesey-Bear. http://garyentsminger.com/ecosim.htm.