Abstract
Arabidopsis thaliana BOR1 is the first boron (B) transporter identified in the living systems. In the rice genome, there are four AtBOR1-like genes, OsBOR1, 2, 3 and 4. We have previously demonstrated that OsBOR4 is a B efflux transporter gene specifically expressed in rice pollen. OsBOR4 heterozygous lines showed abnormal segregation ratio, suggesting the significance of OsBOR4 in rice pollen tube germination/elongation process. To obtain further insights into the mechanisms underlying fertilization defects by osbor4 mutations, we examined if the mutant pollen exhibits morphological changes. The cross section of the pollen of the mutant was similar to those of the wild type. We also determined B concentrations in brown rice of three osbor4 mutants and found that B levels were comparable. These results suggest that osbor4 mutation does not affect B transport to pollen and seeds. We then examined if exogenous B supplementation can rescue segregation defect of osbor4. As reported previously, a OsBOR4 heterozygous lines showed abnormal segregation rate under the normal growth condition in this present study, too. Importantly, this abnormality in segregation was partially rescued by application of six-times higher B concentration to roots, providing further evidence that the fertilization defect of osbor4 is due to the defect in B transport process. Taken together we propose that osbor4 causes defect in B transport process during pollen germination to fertilization.
Keywords: :
Introduction
Boron (B) is an essential micronutrient for vegetative and reproductive development of plants. Rhamnogalacturonan-II (RG-II) acts as a receptor for B in plant cell walls.Citation1-Citation3 A RG-II dimer formation mediated by B is important for the structural maintenance of cell wallsCitation4 and is thus crucial for shoot development and root elongation.Citation4,Citation5 B is also essential for pollen germination and pollen tube growth.Citation6-Citation9 Cross-linking of RG-II by B plays a role in mechanical strength of the pollen tube cell wall.Citation9,Citation10 Extracellular supply of B leads to rapid pollen tube growth in Lilium formosanum.Citation11 These findings suggest the importance of adequate B supply in efficient pollen tube and root growth. The effects of B deficiency are much apparent on reproductive growth than on early vegetative growth in rice.Citation12 In spite of the significant reproductive growth defect under the B deficient condition, importance of B transport within floral organs were still unknown until very recently.
Arabidopsis BOR1 was reported as the first B transporter in animals and plants.Citation13 Similar genes of BOR1 are found in rice genome, OsBOR1, 2, 3 and 4.Citation14 In previous study, we reported that OsBOR4 is specifically expressed in pollen and plays an important role in reproductive processes of rice.Citation15 Significance of OsBOR4 in reproductive processes is highlighted by the abnormal segregation ratio in the progeny of OsBOR4 heterozygous lines. Less numbers of osbor4 homozygous mutants were detected in the progeny of OsBOR4 heterozygous lines than expected from a Mendelian manner, whereas osbor4 mutant pollen grains are viable and mutant plants are fertile. These suggest that pollen grains from homozygous osbor4 mutant are partially defective and less competitive in comparison to wild-type pollen grains during pollen tube germination/elongation process, resulting in less frequency of homozygous plants in the progeny of heterozygous lines. The phenotype that pollen germination was partially but not seriously retarded by osbor4 mutation supported this hypothesis. Since OsBOR4 is an efflux type B transporter, impaired pollen germination and tube growth in osbor4 mutants is likely due to inadequate B supply to pollen tube cell walls. In this report, we further examined phenotypes of osbor4 mutants to reveal mechanisms underlying partial defect of osbor4 in reproductive processes.
Results and Discussion
Phenotypes of osbor4 mutant in reproductive phase
Previously, we have shown that in osbor4 mutants, the size of flower, grain fertility and structure of anther and pistil were comparable to the wild-type plants.Citation15 To observe detailed phenotypes of floral organs in osbor4 mutants, we first examined the cross section of osbor4 mutant ovary. However, no abnormal development was found in osbor4 mutant ovule and embryo sac (). Then we investigated the structure of anthers. We found that in the middle part of anthers, pollen grains at late pollen mitosis stage were mature and normally developed in pollen sac in both the wild type and osbor4 mutant (). The fibrous structures formed in endothecium cell layer of pollen sac is important for dehiscence of the anthers in the mature pollen stage.Citation16 The driving force for anther dehiscence is derived from fibrous structures in the endothecium cells.Citation16 But no significant alterations were visible in osbor4 fibrous structures in the basal part of anther (). Normal structure of cell layers in osbor4 mutant anther indicates that dehiscence of anther is comparable between wild type and osbor4 mutant. We have previously demonstrated that pollen grains are viable in osbor4 mutants.Citation15 Pistil development in the mutants is also indistinguishable from wild type.Citation15 Thus, taken together, it is reasonable to conclude that OsBOR4 is not crucial for the formation of floral organ and pollen maturation.
Figure 1. Phenotypes of osbor4 floral organs. (A) Cross section of wild-type ovary at the mature ovule stage. OV; ovule, ES; embryo sac, AC; antipodal cells. (B) Cross section of osbor4 mutant ovary at the mature ovule stage. (C) Cross section of wild-type middle part of anther at the mature pollen stage. PS; pollen sac. (D) Cross section of osbor4 mutant middle part of anther at the mature pollen stage. (E) Cross section of wild-type basal part of anther at the mature pollen stage. (F) Cross section of osbor4 mutant basal part of anther at the mature pollen stage. Arrows indicate fibrous structures formed in endothecium cells. Bars = 100μm.
Exogenous B supply partially rescues the abnormal segregation phenotype of an OsBOR4 heterozygous line
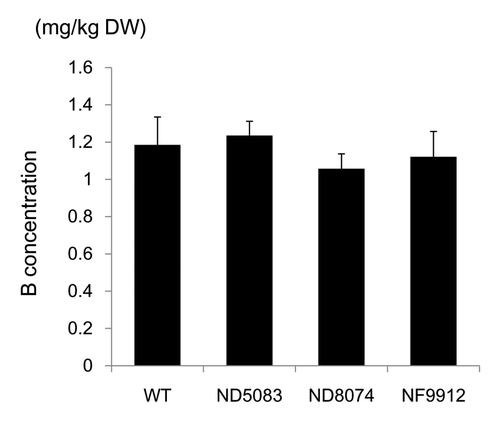
It has been reported that B deficient growth conditions decrease B concentrations in rice plants and impair their pollen germination.Citation12,Citation17 Therefore, internal B deficiency could cause fertilization defect phenotypes of osbor4 mutants. To examine this, we analyzed the B accumulation in seeds of osbor4 mutants (). The B concentrations in brown rice of three osbor4 mutants (ND5083, ND8074 and NF9912) were identical to that of wild type (). We previously demonstrated that B accumulation in leaves is also not affected by osbor4 mutations.Citation15 These findings indicate that OsBOR4 is not involved in B transport from roots to shoots and that to grains.
Figure 2. Boron concentration in the osbor4 mutant seeds. Brown rice B concentration in WT (Nipponbare), ND5083, ND8074 and NF9912 (osbor4 mutants). Plants were grown in soil until ripening stage. Data represent means ± SD (n = 6).
Local B distribution is also known to be important for root and pollen tube growth. Proper formation of B-RG-II complexes appears crucial for plasticity and extensibility of strength of cell wall in pollen tubes or roots. Importance of RG-IIdimer formation mediated by B has been shown in pollen tubeCitation9,Citation10 and root growth.Citation5 In Arabidopsis thaliana, AtBOR2, a paralog of AtBOR1, functions in proper RG-II dimer formation and maintains root elongation under B deficiency, probably through transporting B to apoplast.Citation5 Cross-linked B-RG-II complexes also play an important role in the oscillation of the pollen tube growth.Citation11 Furthermore, in Lilium formosanum, exogenous supply of B causes rapid pollen tube growth in vitro.Citation11 These results suggest the importance of adequate local B supply in efficient pollen tube and root growth.
Considering that OsBOR4 is an efflux transporter of B in pollen grains and important for pollen tube growth,Citation15 it is reasonable to expect that OsBOR4 is required for optimal local B supply to support proper pollen tube growth. The lack of functional OsBOR4 may disrupt proper B supply to apoplast of growing pollen tube and which could retard pollen tube germination/elongation in pollen grains from osbor4 mutants.Citation15 We hypothesized that supplement of B would compensate the retarded pollen tube elongation in osbor4 mutant and would eventually rescue the abnormal segregation ratio in the progeny of OsBOR4 heterozygous plant. To examine this hypothesis, we analyzed the segregation ratio in the progeny of a heterozygous OsBOR4 plant (ND8074) grown with six-times higher B supplement (108μmol/L) compared with our standard hydroponic solution (18μmol/L). As a result of supplying extra B to the plants, segregation ratio of OsBOR4 heterozygous plant was partially rescued (). Compared with the normal B condition, the number of homozygous plants was increased. When the segregation ratios of OsBOR4 heterozygous plants grown with standard hydroponic solution and six-times higher B supplement were defined as the expected value and observed value, respectively, the p-value of Chi-Square Test was 0.0010. Extra B supply to the heterozygous plants may result in providing sufficient B environments for osbor4 mutant pollen tube elongation. This relatively higher B condition for pollen tube growth probably compensates partial defect of osbor4 mutant pollen tube growth and diminishes some disadvantages of mutant pollen against the wild-type pollen in pollen tube germination/elongation process. These results overall suggest the significance of OsBOR4-mediated B transport in pollen tubes during pollen tube germination/elongation process.
Table 1. Segregation of progenies derived from self-pollinated OsBOR4 heterozygous plant (ND8074) under the 18μmol/L and 108μmol/L B condition.
Materials and Methods
Plant materials
Wild-type rice (Oryza sativa L. cv Nipponbare) and Tos17-inserted mutants of the OsBOR4 gene from the Rice Genome Resource CenterCitation18 were used for the experiments. Plants were grown in pots with soil or water culture system with modified Kimura's B nutrient solution under natural conditions. The nutrient solution contained the macronutrients (mM): (NH4)2SO4 (0.35), Na2HPO4·12H2O (0.17), CaNO3·4H2O (0.18), KNO3 (0.54), CaCl2·2H2O (0.19), MgSO4·7H2O (0.47) and Fe-citrate (0.09), and the micronutrients (μM): CuSO4·5H2O (0.16), ZnSO4·7H2O (0.15), Na2MoO4·2H2O (0.10), MnSO4·5H2O (4.60), and H3BO3 (18.0). The pH of this solution was adjusted to 5.7 using MES.
Paraffin sectioning
Plant samples of wild-type and osbor4 were fixed with FAA (formalin: acetic acid: 50% ethanol, 1:1:18)) for 24 h at 4 °C. They were dehydrated in a graded ethanol series and embedded in Paraplast plus (McCormick Scientific). Microtome sections (8 μm thick) were stained with Delafield's hematoxylin.
Determination of B concentration
Brown rice of wild-type and osbor4 were harvested to determine the B concentration. Dried brown rice was digested in 2 mL of nitric acid. After dilution, the concentration of B in the brown rice was analyzed by ICP-MS (model SPQ9700; SII Nano-Technology, Seiko).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research (S) to T.F. (no. 25221202), a Grant-in-Aid for Scientific Research on Innovative Areas to T.F. (nos. 25114502, 25114506, 22119002) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Kobayashi M, Matoh T, Azuma JI. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 1996; 110:1017–20; PMID:12226238; 10.1104/pp.110.3.1017.
- Matoh T, Kawaguchi S, Kobayashi M. Ubiquity of a borate-rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol 1996; 37:636 - 40; http://dx.doi.org/10.1093/oxfordjournals.pcp.a028992
- O’Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 2004; 55:109 - 39; http://dx.doi.org/10.1146/annurev.arplant.55.031903.141750; PMID: 15377216
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 2001; 294:846 - 9; http://dx.doi.org/10.1126/science.1062319; PMID: 11679668
- Miwa K, Wakuta S, Takada S, Ide K, Takano J, Naito S, Omori H, Matsunaga T, Fujiwara T. Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiol 2013; 163:1699 - 709; http://dx.doi.org/10.1104/pp.113.225995; PMID: 24114060
- Cheng C, Rerkasem B. Effects of Boron on pollen viability in wheat. Plant Soil 1993; 155-156:313 - 5; http://dx.doi.org/10.1007/BF00025045
- Huang LB, Pant J, Dell B, Bell RW. Effects of boron deficiency on anther development and floret fertility in wheat (Triticum aestivum L-'Wilgoyne'). Ann Bot (Lond) 2000; 85:493 - 500; http://dx.doi.org/10.1006/anbo.1999.1095
- Wang Q, Lu L, Wu X, Li Y, Lin J. Boron influences pollen germination and pollen tube growth in Picea meyeri.. Tree Physiol 2003; 23:345 - 51; http://dx.doi.org/10.1093/treephys/23.5.345; PMID: 12615549
- Iwai H, Hokura A, Oishi M, Chida H, Ishii T, Sakai S, Satoh S. The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proc Natl Acad Sci U S A 2006; 103:16592 - 7; http://dx.doi.org/10.1073/pnas.0605141103; PMID: 17053077
- Delmas F, Séveno M, Northey JG, Hernould M, Lerouge P, McCourt P, Chevalier C. The synthesis of the rhamnogalacturonan II component 3-deoxy-D-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. J Exp Bot 2008; 59:2639 - 47; http://dx.doi.org/10.1093/jxb/ern118; PMID: 18503041
- Holdaway-Clarke TL, Weddle NM, Kim S, Robi A, Parris C, Kunkel JG, Hepler PK. Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J Exp Bot 2003; 54:65 - 72; http://dx.doi.org/10.1093/jxb/erg004; PMID: 12456756
- Uraguchi S, Fujiwara T. Significant contribution of boron stored in seeds to initial growth of rice seedlings. Plant Soil 2011; 340:435 - 42; http://dx.doi.org/10.1007/s11104-010-0614-9
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. Arabidopsis boron transporter for xylem loading. Nature 2002; 420:337 - 40; http://dx.doi.org/10.1038/nature01139; PMID: 12447444
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T. Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 2007; 19:2624 - 35; http://dx.doi.org/10.1105/tpc.106.049015; PMID: 17675406
- Tanaka N, Uraguchi S, Saito A, Kajikawa M, Kasai K, Sato Y, Nagamura Y, Fujiwara T. Roles of pollen-specific boron efflux transporter, OsBOR4, in the rice fertilization process. Plant Cell Physiol 2013; 54:2011 - 9; http://dx.doi.org/10.1093/pcp/pct136; PMID: 24068795
- Matsui T, Omasa K, Horie T. Mechanism of Anther Dehiscence in Rice (Oryza sativa L.). Ann Bot (Lond) 1999; 84:501 - 6; http://dx.doi.org/10.1006/anbo.1999.0943
- Lordkaew S, Konsaeng S, Jongjaidee J, Dell B, Rerkasem B, Jamjod S. Variation in responses to boron in rice. Plant Soil 2013; 363:287 - 95; http://dx.doi.org/10.1007/s11104-012-1323-3
- Miyao A, Tanaka K, Murata K, Sawaki H, Takeda S, Abe K, Shinozuka Y, Onosato K, Hirochika H. Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. Plant Cell 2003; 15:1771 - 80; http://dx.doi.org/10.1105/tpc.012559; PMID: 12897251
