Abstract
Plant growth and development are tightly regulated by both plant growth substances and environmental factors such as temperature. Taking into account the above, it was reasonable to point out that indole-3-acetic acid (IAA), the most abundant type of auxin in plants, could be involved in temperature-dependent growth of plant cells. We have recently shown that growth of maize coleoptile segments in the presence of auxin (IAA) and fusicoccin (FC) shows the maximum value in the range 30-35˚C and 35-40˚C, respectively. Furthermore, simultaneous measurements of growth and external medium pH indicated that FC at stressful temperatures was not only much more active in the stimulation of growth, but was also more effective in acidifying the external medium than IAA. The aim of this addendum is to determine interrelations between the action of IAA and FC (applied together with IAA) on growth and medium pH of maize coleoptile segments incubated at high temperature (40˚C), which was optimal for FC but not for IAA.
Addendum to: Karcz W, Burdach Z. Effect of temperature on growth, proton extrusion and membrane potential in maize (Zea mays L.) coleoptile segments. Plant Growth Regul 2007; 52:141-50.
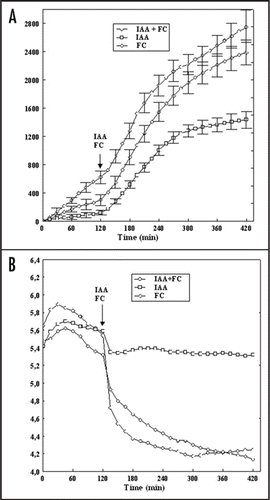
A well studied aspect of auxin action especially in maize coleoptile, is its effect on cell elongation, proton extrusion and membrane potential.Citation1–Citation7 It is now generally agreed that indole-3-acetic acid (IAA), as the principal regulator of plant elongation growth, causes (i) acceleration of elongation growth as compared to endogenous growth, (ii) enhancement of proton extrusion as compared to auxin—free medium, and (iii) transient depolarization followed by a slow hyperpolarization of membrane potential. According to the “acid growth theory” of elongation growth,Citation8–Citation11 auxin induced cell wall acidification provides favorable conditions for cell wall loosening, a requirement for cell elongation. At least in maize coleoptile segments, auxin induced cell wall acidification is mediated by increased activity and/or amount of the PM H+-ATPase.Citation11,Citation12 In the case of fusicoccin, which mimics the effect of auxin in many respects,Citation13 it was shown that FC-binding site arises from interaction of the 14-3-3 protein dimmer with the C-terminal autoinhibitory domain of the H+-ATPase and that FC stabilizes this complex.Citation14–Citation18 It should be pointed out that in spite of abundant literature on the mechanism through which IAA or FC control growth of grass coleoptiles, little is know how these substances work at extreme temperatures. Over the past decade, the involvement of 14-3-3 proteins in plant stress responses has often been suggested.Citation19 For example, work by Chelysheva et al.,Citation20 and Babakov et al.,Citation21 demonstrated that under low temperature and high osmolarity conditions, 14-3-3 proteins interact with the C-terminal autoinhibitory domain of the PM H+-ATPase activating the proton pump that play a key role in stress responses in higher plants. We have recently shownCitation22 that FC at 40°C induced maximal growth whereas growth observed at the same temperature in the presence of IAA was reduced by 33% compared to the maximal value at 30°C. It was also foundCitation22 that at 40°C the kinetics of the pH change differed significantly for both growth substances; the segments treated with IAA at 40°C were virtually not able to acidify the external medium, whereas FC at this temperature caused practically maximal acidification. In this addendum we have shown that application of FC together with IAA conteracted the inhibitory effect of high temperature (40°C) on IAA-induced growth and proton extrusion in maize coleoptile segments (). For example, the total IAA-induced elongation growth of coleoptile segments at 40°C was 1438.1 ± 134.5 µm cm−1 (mean ± SE, n = 11) while elongation of 2747.4 ± 269.7 µm cm−1 (mean ± SE, n = 11) was observed in IAA applied together with FC (). The data in indicate that coleoptile segments incubated at 40°C (over 2 h), without growth substances (control) characteristically changed the pH of the medium: usually within the first 30–45 min an increase of pH (by ca. 0.5 pH unit) was observed, followed by a slow decrease of pH. When IAA or FC was added (after 2 h of segment's incubation in control medium), an additional decrease of pH was observed. As can be seen in , FC added at 40°C was much more effective in acidification of the medium, as compared to IAA. For FC, 5h after its addition, the pH of the incubation medium dropped to pH 4.2, whereas for IAA the pH was only 5.4. However, addition of IAA together with FC at 40°C dropped medium pH approximately to the same value as was observed in the presence of FC only.
In conclusion, the results presented in this addendum provide further evidence that FC on the receptor level is much more effective than IAA.
Abbreviations
| IAA | = | indole-3-acetic acid |
| FC | = | fusicoccin |
Figures and Tables
Figure 1 Effect of high temperature (40°C) on growth (A) and medium pH (B) of maize coleoptile segments incubated in the presence of IAA (10 µM) and FC (1 µM). The growth of a stack of 21 segments, expressed as elongation (µm cm−1), was measured simultaneously with medium pH at 40°C. After preincubation (over 2 h) of the coleoptile segments in control medium, IAA and FC was added (arrow). Values are means of 11 independent experiments. Bars indicate ± SE. In the case of medium pH SE did not exceed 8%.
Addendum to:
References
- Kutschera U, Schopfer P. Evidence against the acid-growth theory of auxin action. Planta 1985; 163:483 - 493
- Karcz W, Stolarek J, Pietruszka M, Malkowski E. The dose-response curves for IAA induced elongation growth and acidification of the incubation medium of Zea mays L. coleoptile segments. Physiol Plant 1990; 80:257 - 261
- Lüthen H, Bigdon M, Böttger M. Reexamination of the acid growth theory of auxin action. Plant Physiol 1990; 93:931 - 939
- Felle H, Peters WS, Palme K. The electrical response of maize to auxins. Biochim Biophys Acta 1991; 1064:199 - 204
- Karcz W, Stolarek J, Lekacz H, Kurtyka R, Burdach Z. Comparative investigation of auxin and fusicoccin-induced growth and H+-extrusion in coleoptile of Zea mays L. Acta Physiol Plant 1995; 17:3 - 8
- Claussen M, Lüthen H, Böttger M. Inside or outside? Localization of the receptor relevant to auxin-induced growth. Physiol Plant 1996; 98:861 - 867
- Karcz W, Burdach Z. A comparison of the effects of IAA and 4-Cl-IAA on growth, proton secretion and membrane potential in maize coleoptile segments. J Exp Bot 2002; 53:1089 - 1098
- Rayle DL, Cleland RE. Enhancement of wall loosening and elongation by acid solutions. Plant Physiol 1970; 46:250 - 253
- Rayle DL, Cleland RE. The acid-growth theory of auxin induced cell elongation is alive and well. Plant Physiol 1992; 99:1271 - 1274
- Hager A, Menzel H, Krauss A. Versuchte und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum. Planta 1971; 100:1 - 15
- Hager A, Debus G, Edel HG, Stransky H, Serrano R. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma membrane H+-ATPase. Planta 1991; 185:527 - 537
- Frias I, Caldeira MT, Pérez Castiñeira JR, Navarro Aviñó JP, Culiañez Maciá FA, Kuppinger O, Stransky H, Pagés M, Hager A, Serrano R. A major isoform of the maize plasma membrane H+-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell 1996; 8:1533 - 1544
- Marrè E. Fusicoccin: a tool in plant physiology. Ann Rev Plant Physiol 1979; 30:273 - 288
- Jahn T, Fuglesang AT, Olsson A, Brüntrup IM, Collinge DB, Volkmann D, Sommarin M, Palmgren MG, Larsson C. The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H+-ATPase. Plant Cell 1997; 9:1805 - 1814
- Baunsgaard L, Fuglsang AT, Jahn T, Korthout HAAJ, De Boer AH, Palmgren MG. The 14-3-3 proteins associate with the plasma membrane H+-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J 1998; 13:661 - 671
- Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr(946)-thr-Val and requires phosphorylation of Thr(947). J Biol Chem 1999; 274:36774 - 36780
- Oecking C, Hagemann K. Association of 14-3-3 proteins with the C-terminal autoinhibitory domain of the plant plasma membrane H+-ATPase generates a fusicoccin-binding complex. Planta 1999; 207:480 - 482
- Würtele M, Jelich Ottmann Ch, Wittinghofer A, Oecking C. Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J 2003; 22:987 - 994
- Roberts MR, Salinas J, Collinge DB. 14-3-3 proteins and the response to abiotic stress. Plant Mol Biol 2002; 1031:1031 - 1039
- Chelysheva VV, Smolenskaya IN, Trofimova MC, Babakov AV, Muromtsev GS. Role of the 14-3-3 proteins in the regulation of H+-ATPase activity in the plasma membrane of suspension-cultured sugar beet cells under cold stress. FEBS Lett 1999; 456:22 - 26
- Babakov AV, Chelysheva VV, Klychnikov OI, Zorinyanz SE, Trofimova MS, de Boer AH. Involvement of 14-3-3 proteins in the osmotic regulation of H+-ATPase in plant plasma membranes. Planta 2000; 211:446 - 448
- Karcz W, Burdach Z. Effect of temperature on growth, proton extrusion and membrane potential in maize (Zea mays L.) coleoptile segments. Plant Growth Regul 2007; 52:141 - 150