Abstract
Environmental and developmental signals can elicit differential activation of membrane proton (H+) fluxes as one of the primary responses of plant and fungal cells. In recent work,1 we could determine that during the presymbiotic growth of arbuscular mycorrhizal (AM) fungi specific domains of H+ flux are activated by clover root factors, namely host root exudates or whole root system. Consequently, activation on hyphal growth and branching were observed and the role of plasma membrane H+-ATPase was investigated. The specific inhibitors differentially abolished most of hyphal H+ effluxes and fungal growth. As this enzyme can act in signal transduction pathways, we believe that spatial and temporal oscillations of the hyphal H+ fluxes could represent a pH signature for both early events of the AM symbiosis and fungal ontogeny.
Addendum to: Ramos AC, Façanha AR, Feijó JA. Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol 2008; 178:177-88.
The 450-million-year-old symbiosis between the majority of land plants and arbuscular mycorrhizal (AM) fungi is one of the most ancient, abundant and ecologically important symbiosis on Earth.Citation2,Citation3
The development of AM interaction starts before the physical contact between the host plant roots and the AM fungus. The hyphal growth and branching are induced by the root factors exudated by host plants, followed by the formation of appressorium leading to the hyphal penetration in the root system. These root factors seems to be specifically synthesized by host plants, since exudates from non-host plants are not able to promote neither hyphal differentiation nor appressorium formation.Citation4,Citation5 Most root exudates contain several host signals or better, active compounds including flavonoidsCitation6,Citation19 and strigolactones,Citation7,Citation8 however many of them are not yet known.
Protons (H+) may have an important role on the fungal growth and host signal perception.Citation1 In plant and fungal cells, H+ can be pumped out through two different mechanisms: (1) the activity of the P-type plasma membrane (PM) H+-ATPaseCitation9 and (2) PM redox reactions.Citation10 The proportional contribution from both mechanisms is not known, but in most plant cells the PM H+-ATPase seems to be the major responsible by the H+ efflux across plasma membrane. AM Fungal cells also energize their PM using P-type H+-pumps quite similar to the plant ones. Indeed, some genes codifying isoforms of P-type H+-ATPase have been isolated of AM fungi,Citation11–Citation13 and AM fungal ATP hydrolysis activity was shown by cytochemistry, localized mainly in the first 70 µm from the germ tube tip.Citation14 This structural evidence correlates with data obtained by H+-specific vibrating probe ( and B), which indicates that the H+ efflux in Gigaspora margarita is more intense in the subapical region of the lateral hyphaeCitation1 (). Furthermore, the correlation between the cytosolic pH profile previously obtained by Jolicoeur et al.,Citation15 with the H+ efflux pattern (erythrosine-dependent), seems to clearly indicate that an active PM H+-ATPase takes place at the subapical hyphal region. Using orthovanadate, we could show that those H+ effluxes are susceptible mainly in the subapical region, but no effect in the apical was found.Citation1 Recently, a method to use fluorescent marker expression in an AM fungus driven by arbuscular mycorrhizal promoters was published.Citation31 It could be adjusted as an alternative to measure “in vivo” PM H+-ATPase expression in AM fungal hyphae and their responses to root factors.Citation31
The H+ electrochemical gradient generated by PM H+-ATPases provides not only driving force for nutrient uptake,Citation9,Citation16 but also can act as an intermediate in signal transduction pathways.Citation18 The participation of these H+ pumps in cell polarity and tip growth of plant cells was recently reported,Citation27 addressing their crucial role on apical growth.Citation28 Naturally, in the absence of root factors the AM fungi have basal metabolicCitation8,Citation21–Citation23 and respiratory activity.Citation24 However when root signals are recognized and processed by AM fungal cells they might become activated.Citation22 We thus searched for pH signatures that could reflect the alterations on fungal metabolism in response to external stimuli. In fact, preliminary analyses from our group demonstrate that AM fungal hyphae increase their H+ efflux in response not only to root exudates recognition, but also to other root factors (). The incubation for 30 min of AM fungal hyphae with several root factors induces hyphal H+ efflux similar to the response to intact root system (5 days of incubation). The major increases were found with 1% CO2 (750%) followed by root cell wall proteins (221%), root exudates (130%) and synthetic strigolactone (5%) (). Those stimulations could define the transition from the state without root signals to the presymbiotic developmental stage (). In the case of CO2, the incorporation of additional carbon could represent a new source of energy, since CO2 dark fixation takes place in Glomus intraradices germ tubes.Citation22,Citation25
Interestingly, after the treatment with synthetic strigolactone (10−5 M GR24), no significant stimulation was found compared to the remaining factors (). It opens the question if the real effect of strigolactone is restrict to hyphal branching and does not intervene in very fast response pathways. Likewise, strigolactones need additional time to exhibit an effect, as recently discussed by Steinkellner et al.,Citation26 However, at the moment, no comprehensive electrophysiological analyses are presently available separating the effects of strigolactone and some flavonoids in AM fungal hyphae.
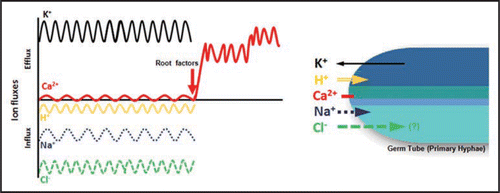
The next target of our work is the study of ionic responses of single germ tubes or primary hyphae to root factors (). As reported by Ramos et al.,Citation1 we have been observing that the pattern of ion fluxes at the apical zone of primary hyphae is differentiated from secondary or lateral hyphae. In the primary, two interesting responses were detected in the absence of root factors: (1) a “dormant Ca2+ flux” and (2) Cl− or anion fluxes at the same direction of H+ ions, suggesting a possible presence of H+/Cl− symporters at the apex, similarly to what occurs in root hairs ().Citation30 In the presence of root factors such as root exudates the stimulated influxes of Cl− (anion), H+, Na+ and effluxes of K+ and Ca2+ are activated. It can explain why the AM fungi hyphal tips are depolarizedCitation20,Citation29 during the period without root signals—“asymbiosis”—as long as K+ efflux and H+ influx occur simultaneously. Indeed, H+ as well as Ca2+ ions may act as second messengers, where extra and intracellular transient pH changes are preconditions for a number of processes, including gravity responses and possibly in plant-microbe interactions.Citation17,Citation30
Clearly, further data on the mechanism of action of signaling molecules such as strigolactones over the signal transduction and ion dynamics in AM fungi will be very important to improve our understanding of the molecular bases of the mycorrhization process. Future studies are necessary in order to provide basic knowledge of the ion signaling mechanisms and their role on the response of very important molecules playing at the early events of AM symbiosis.
Figures and Tables
Figure 1 (A) H+ flux profile along growing secondary hyphae of G. margarita in the presence (open squares) or absence (closed squares) of erythrosin B and its correlation with cytosolic pH (pHc) data described by Jolicoeur et al.,Citation15 (dotted line). Dotted area depicts the region with higher susceptibility to erythrosin B. (B) ion-selective electrode near to AM fungal hyphae. (C) Stimulation on hyphal H+ efflux after incubation with root factors or whole root system. R, roots; RE, root exudates; CO2, carbon dioxide; CWP, cell wall proteins; GR24, synthetic strigolactone. The medium pH in all treatment was monitored and remained about 5.7, including with prior CO2 incubation. Means followed by the same letter are statistically equal by Duncan's test at p < 5%.
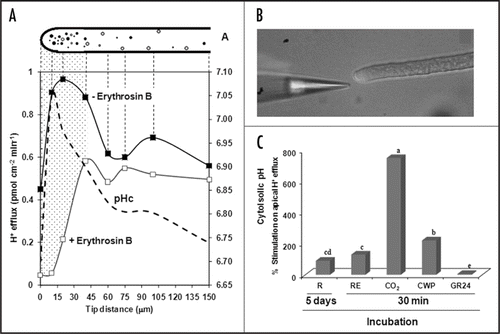
Figure 2 Ion dynamics in the apex of primary hyphae of arbuscular mycorrhizal fungi. It represents the Stage 1 described in Ramos et al.Citation1 After treatment with root factors, an activation of Ca2+ efflux is observed at the hyphal apex.
Addendum to:
References
- Ramos AC, Façanha AR, Feijó JA. A proton (H+) flux signature of the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol 2008; 178:177 - 188
- Remy W, Taylor TN, Hass H, Kerp H. 4-Hundred-million-year-old vesicular-arbuscular mycorrhizae. Proc Natl Acad Sci USA 1994; 91:11841 - 11843
- Taylor TN, Remy W, Hass H, Kerp H. Fossil arbuscular mycorrhizae from the early devonian. Mycologia 1995; 87:560 - 573
- Smith SE, Read DJ. Mycorrhizal symbiosis 2007; London
- Giovannetti M, Sbrana C, Citernesi AS, Avio L. Analysis of factors involved in fungal recognition responses to host derived signals by arbuscular mycorrhizal fungi. New Phytol 1996; 133:65 - 71
- Nair MG, Safir GR, Siqueira JO. Isolation and identification of vesicular-arbuscular mycorrhiza-stimulatory compounds from clover (Trifolium repens roots. Appl Environ Microbiol 1992; 57:434 - 439
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005; 435:824 - 827
- Besserer A, Puech-Pages V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Sejalon Delmas N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. Plos Biol 2006; 4:1239 - 1247
- Palmgren MG. Plant plasma membrane H+-ATPases: Powerhouses for nutrient uptake. Ann Rev Plant Physiol Plant Mol Biol 2001; 52:817 - 845
- Kim YC, Wikstrom M, Hummer G. Kinetic models of redox-coupled proton pumping. Proc Natl Acad Sci USA 2007; 104:2169 - 2174
- Ferrol N, Barea JM, Azcon Aguilar C. The plasma membrane H+-ATPase gene family in the arbuscular mycorrhizal fungus Glomus mosseae. Curr Genetics 2000; 37:112 - 118
- Requena N, Breuninger M, Franken P, Ocon A. Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant Physiol 2003; 132:1540 - 1549
- Corradi N, Kuhn G, Sanders IR. Monophyly of beta-tubulin and H+-ATPase gene variants in Glomus intraradices: consequences for molecular evolutionary studies of AM fungal genes. Fungal Genetics Biol 2004; 41:262 - 273
- Lei J, Bécard G, Catford JG, Piché Y. Root factors stimulate P32 uptake and plasmalemma atpase activity in vesicular arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytol 1991; 118:289 - 294
- Jolicoeur M, Germette S, Gaudette M, Perrier M, Bécard G. Intracellular pH in arbuscular mycorrhizal fungi: a symbiotic physiological marker. Plant Physiol 1998; 116:1279 - 1288
- Felle HH. pH: Signal and messenger in plant cells. Plant Biol 2001; 3:577 - 591
- Feijó JA, Costa SS, Prado AM, Becker JD, Certal AC. Signalling by tips. Curr Opin Plant Biol 2004; 7:589 - 598
- Xing T, Higgins VJ, Blumwald E. Regulation of plant defense response to fungal pathogens: Two types of protein kinases in the reversible phosphorylation of the host plasma membrane H+-ATPase. Plant Cell 1996; 8:555 - 564
- Chabot S, Belrhlid R, Chenevert R, Piché Y. Hyphal growth promotion in vitro of the VA mycorrhizal fungus, Gigaspora margarita Becker and Hall, by the activity of structurally specific flavonoid compounds under CO2 enriched conditions. New Phytol 1992; 122:461 - 467
- Berbara RLL, Morris BM, Fonseca H, Reid B, Gow NAR, Daft MJ. Electrical currents associated with arbuscular mycorrhizal interactions. New Phytol 1995; 129:433 - 438
- Bécard G, Piché Y. Fungal growth-stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl Environ Microbiol 1989; 55:2320 - 2325
- Ramos AC, Façanha AR, Feijó JA. Varma A, Hock B. Ion dynamics during the polarized growth of arbuscular mycorrhizal fungi: from presymbiosis to symbiosis. Mycorrhiza: Biology, Genetics, Novel Endophytes and Biotechnology 2008; Germany Springer-Verlag (in press)
- Buee M, Rossignol M, Jauneau A, Ranjeva R, Bécard G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol Plant-Microb Interact 2000; 13:693 - 698
- Tamasloukht M, Sejalon Delmas N, Kluever A, Jauneau A, Roux C, Bécard G, Franken P. Root factors induce mitochondrial-related gene expression and fungal respiration during the developmental switch from asymbiosis to presymbiosis in the arbuscular mycorrhizal fungus Gigaspora rosea. Plant Physiol 2003; 131:1468 - 1478
- Lammers PJ, Jun J, Abubaker J, Arreola R, Gopalan A, Bago B, Hernandez Sebastia C, Allen JW, Douds DD, Pfeffer PE, Shachar Hill Y. The glyoxylate cycle in an arbuscular mycorrhizal fungus. Carbon flux and gene expression. Plant Physiol 2001; 127:1287 - 1298
- Steinkellner S, Lendzemo V, Langer I, Schweiger P, Khaosaad T, Toussaint JP, Vierheilig H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 2007; 12:1290 - 1306
- Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguéz Léon J, Wu HM, Cheung AY, Feijó JA. Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell 2008; http://dx.doi.org/10.1105/tpc.106.047423
- Boavida LC, Vieira AM, Becker JD, Feijó JA. Gametophyte interaction and sexual reproduction: how plants make a zygote. Int J Dev Biol 2005; 49:615 - 632
- Ayling SM, Smith SE, Smith FA. Transmembrane electric potential difference of germ tubes of arbuscular mycorrhizal fungi responds to external stimuli. New Phytol 2000; 147:631 - 639
- Felle H. The H+/Cl− symporter in root-hair cells of Sinapis alba. Plant Physiol 1994; 106:1131 - 1136
- Helber N, Requena N. Expression of the fluorescence markers DsRed and GFP fused to a nuclear localization signal in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 2008; 177:537 - 548