Abstract
Symplastic domains in plants are defined by spatial limitations on cell-to-cell communication through plasmodesmata (Pds) and establish tissue boundaries necessary for metabolic and developmental programming. With the exception of the physical closure of Pds by callose, the cues and the processes for creating symplastic domains remain poorly understood. Recently, we identified a novel family of eight proteins, called Pd-located protein 1 (PDLP1). These proteins span the plasma membrane within Pds and likely form part of a signal transduction system that perceives external signals to regulate molecular trafficking between cells. For two members of this family that have high expression in the shoot apex we show that they have defined and partially overlapping tissue-specific expression patterns that correlate in part with previously defined symplastic domains. The importance of non-cell-autonomous proteins in shoot development and of the spatial rules that govern leaf and floral development highlight the need to have a clearer understanding of symplastic domains.
Addendum to:Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol 2008; 6:180-90.
The complex body of plants is divided into physically and/or functionally distinct tissues, which in many cases, are sustained through the regulation of symplastic cell-to-cell communication through Pds. Hence, for example, the complex organisation of the vascular tissues is associated with different forms and frequencies of Pds at different tissue/cellular boundaries.Citation1 Most obviously, the boundary between the phloem companion cell and the sieve element is bridged by specialised deltoid Pds that impose molecular size constraints and regulate the flow of carbohydrates into the phloem.Citation1 In other tissues the functional boundaries are less obvious and have only been identified through the use of symplastically restricted fluorescent markers introduced by microinjection,Citation2 through cell damageCitation3 or through vascular translocation (e.g. HPTS: 8-hydroxypyrene 1,3,6 trisulfonic acid or CF: carboxy fluoresceinCitation4).
The body of the shoot apex is divided into distinct cell layers called the tunica and the corpus. The tunica usually consists of the two outermost single cell layers (the epidermal L1 layer and sub-epidermal L2), which exclusively undergo anticlinal divisions. The corpus is represented by the mass of cells in inner layers (L3) where divisions occur in all planes. Since Pds are formed primarily on the developing cell plate, primary Pds are aligned in the division plane. However, all the cells of the meristem are connected symplastically. Hence Pds on walls connecting L1 and L2, and L2 and the corpus (L3), probably have a distinct ontogeny independent of cell division (secondary Pds). That these provide symplastic connectivity is apparent from the non-cell-autonomous properties of the homeobox protein, KNOTTED1 (KN1). KN1 is expressed in the L2 and L3 zones but the protein is distributed through layers L1 to L3,Citation5 a non-cell-autonomous trafficking property that depends upon the homeobox domain.Citation6
Identifying symplastic domains within a mass of relatively undifferentiated tissue in the shoot apex is complicated by the difficulties of microinjecting fluorescent dyes below the tunica. However, using this approach for just the L1, Rinne and van de SchootCitation2 defined the tunica central and peripheral zones as distinct symplastic domains in the birch apical meristem. These domains appeared not to be maintained by callose deposition at the neck region of Pds, although callose was involved in symplastic closure across the meristem during morphogenetic changes induced by short photoperiods.
From a proteomic survey of Arabidopsis suspension culture cell walls, we recently identified a family of eight genes encoding novel Pd proteins, called Pd-located proteins 1 (PDLP1).Citation7 We showed that these proteins span the plasma membrane within Pds and, with altered expression of the genes, have the potential to regulate Pd trafficking. The sequences of the eight PDLP1 proteins are similar (range 23–78%) raising the possibility of functional redundancy where there would be spatial overlap in the pattern of tissue-specific gene expression. The public transcriptomic data show diverse patterns of gene expression for these genes () with At5g43980 (PDLP1a) being most abundant in cell suspensions, the source tissue for our proteomic analysis. Other members of the family were variously expressed strongly in roots, senescent leaves, stem nodes, siliques, seeds and pollen. Of particular interest to us in the context of the symplastic organisation of the shoot apex were At2g33330 and At1g04520, which were strongly expressed there, although not uniquely.
To investigate the precise tissue distribution of the proteins encoded by At2g33330 and At1g04520 we made GFP fusions to the C-terminal end of the coding sequence and expressed them using the homologues promoter regions (∼1.5 kbp) upstream of the transcriptional start site. The constructs were transformed into Arabidopsis Col-0. Homozygous transgenic seed were germinated and the early inflorescence meristem dissected for examination by confocal microscopy.
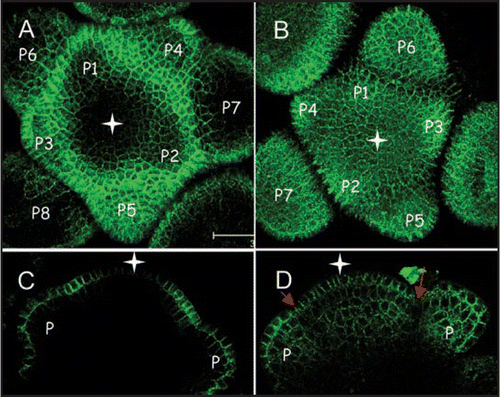
At2g33330.GFPfusion proteinwas mainly expressed in the outermost L1 layer of the shoot apical meristem and in the epidermis of bulging floral primordia ( and C). Within the L1, At2g33330.GFP expression was restricted to the peripheral zone leaving the central zone free of GFP signal ( and C, white arrow). Expression in the epidermis of floral primordia changed through development from being weakly expressed in the youngest the floral organs, with possibly a stronger expression on the abaxial side (P1, P2 stage) to being relatively strongly expressed in both the adaxial and abaxial epidermis at later stages (). In line with the Pd labelling in leaf tissues seen with PDLP1.GFP fusions,Citation7 most of the labelling occurred on the wall. Labelling was found evenly distributed throughout walls with the exception of the outer wall which showed weak labelling. In the absence of an outer connecting cell layer the outer wall does not contain Pds. Thus, the distribution of At2g33330 clearly differentiated the L1 layer from the underlying cell layers as well as the distinct central and peripheral zones within the L1.
The plasmodesmal protein encoded by At1g04520 was uniformly expressed within the inflorescence meristem ( and D) with the exception of a boundary zone between floral primordia and the meristem where the expression was weaker (grey arrows).
To assess the functional significance of these genes in shoot development, insertional knock-out lines for each were obtained (SALK_118839; At1g04520 and SALK_016528; At2g01660) and double knock-out lines also generated. All of these lines grew normally showing no detectable abnormalities in leaf or flower development under short or long day growth conditions, most likely indicating functional redundancy with other PDLP1 genes. Notably, At3g60720, At5g37660 and At2g01660 show low levels of expression in the apical meristem (). Although we do not know the precise biochemical function of the PDLP1 proteins,Citation7 it seems unlikely that the correlation between the distribution of At2g33330 and previously identified symplastic domains in the tunica is coincidence. This raises the possibility for these proteins to be used as potential markers for such domains that otherwise had only been identified by invasive dye tracking experiments. As more Pd proteins are identified a detailed description of their tissue and cell specific patterns of expression and distribution will help to provide a comprehensive picture of the contribution of Pds to the spatial and functional segregation within plant tissues.
Abbreviations
| Pd | = | plasmodesma/plasmodesmal |
| Pds | = | plasmodesmata |
| PDLP1 | = | Pd-located protein 1 |
| KN1 | = | knotted1 |
| GFP | = | green fluorescent protein |
Figures and Tables
Figure 1 Plant-wide tissue expression profiles for the PDLP1 family. (A) Heat-map representation of the public expression data (www.genevestigator.ethz.ch) for the Arabidopsis PDLP1 gene family. High expression in the shoot apex was seen for At2g33330 and At1g04520 (red boxes). (B) Tissues sources tabulated in (A).
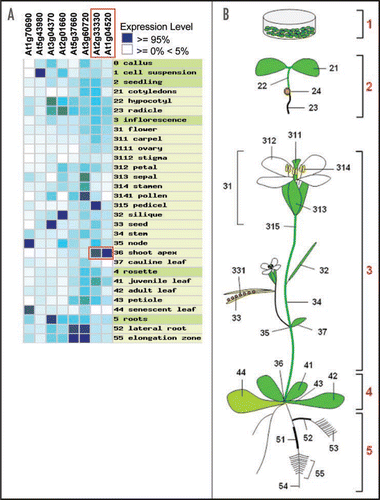
Figure 2 Expression pattern of two members of the PDLP1 plasmodemal protein family at the inflorecence shoot apical meristem of Arabidopsis. (A and C) At2g33330:GFP. (B and D) At1g04520:GFP. (A and B) GFP fluorescence seen from the top view of Arabidopsis inflorescence meristems (maximum projection of transverse confocal scans). (C and D) GFP fluorescence in longitudinal confocal sections through Arabidopsis inflorescence meristems. White star indicates central zone position. Grey arrows indicate the boundary between floral primordia (P; P1–P7) and the meristem. Bars = 20 µm.
Acknowledgements
The John Innes Centre is grant aided by the UK Biotechnology and Biological Science Research Council.
Addendum to:
References
- Turgeon R, Medville R. Phloem loading. A reevaluation of the relationship between plasmodesmatal frequencies and loading strategies. Plant Physiol 2004; 136:3795 - 3803
- Rinne PL, van der Schoot C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 1998; 125:1477 - 1485
- Kim I, Zambryski PC. Cell-to-cell communication via plasmodesmata during Arabidopsis embryogenesis. Curr Opin Plant Biol 2005; 8:593 - 599
- Gisel A, Hempel FD, Barella S, Zambryski P. Leaf-to-shoot apex movement of symplastic tracer is restricted coincident with flowering in Arabidopsis. Proc Natl Acad Sci USA 2002; 99:1713 - 1717
- Kim JY, Yuan Z, Jackson D. Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development 2003; 130:4351 - 4362
- Kim JY, Rim Y, Wang J, Jackson D. A novel cell-to-cell trafficking assay indicates that the KNOX homeodomain is necessary and sufficient for intercellular protein and mRNA trafficking. Genes Dev 2005; 19:788 - 793
- Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biology 2008; 6:180 - 190