Abstract
In a recent paper, we reported that both ethylene and jasmonic acid (JA) are important for selenium (Se) resistance in Arabidopsis.1 Elevated levels of reactive oxygen species were associated with ethylene and JA production in a Se-resistant Arabidopsis ecotype. Here, we further discuss the functions of these phytohormones, and their possible interactions, in plant Se resistance and –accumulation, placing our data in a broader perspective of other recently published papers.
Addendum to: Tamaoki M, Freeman JL, Pilon-Smits EAH. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis thaliana. Plant Physiol 2008;146:1219-30.
Selenium (Se) is a naturally occurring element commonly found in sedimentary rocks formed during the Carboniferous to Quaternary Periods.Citation2 Irrigation of Se-rich soils leads to selenate leaching in shallow groundwater.Citation3 In addition, selenite is a common contaminant in oil-refinery wastewater.Citation4 The accumulation of Se in surface water or soil can become a source of toxicity for plants.Citation5 Se is chemically similar to sulfur (S) and can be metabolized by S metabolic pathways. Plants take up selenate, the most common soluble form of Se in soil, inadvertently via sulfate transporters, and assimilate it into selenocysteine and selenomethionine.Citation5 Nonspecific replacement of the two essential S amino acids cysteine and methionine by these Se analogues in proteins is toxic.Citation5,Citation6 For this reason, much of the research on plant Se resistance has focused on Se interactions with S metabolism.
Recently, we obtained evidence for the involvement of defense-related phytohormones in the acquisition of Se resistance in plants.Citation7 In a transcriptome study performed to identify selenate-responsive genes, the expression of many ethylene and/or jasmonic acid (JA) responsive genes was induced by selenate treatment. Induction of many of these same ethylene and/or JA responsive genes was also observed in selenite-treated plants.Citation1 These include the gene encoding allene oxide synthase (AOS), a key enzyme in JA biosynthesis,Citation8 and known to be induced by MeJA.Citation9 Another selenite-induced gene was PDF1.2 which encodes a plant defensin that requires concomitant triggering of the ethylene and JA pathway for its induction.Citation10 Transgenic AOS promoter::GUS and PDF1.2 promoter::GUS plants exhibited GUS activity in leaves of selenite-treated plants () but not in those of non-treated plants (), confirming that these genes are Se-regulated. Furthermore, selenite treatment led to enhance ethylene generation and JA accumulation, particularly in the selenite-resistant ecotype Col-0.Citation1 Together, these results suggest that Se treatment triggers ethylene and JA production and responses. The importance of ethylene and JA for Se resistance in plants was further investigated using Arabidopsis mutants deficient in an aspect of ethylene- or JA-biosynthesis or signaling (acs6, ein2 and jar1). These mutants showed less resistance to selenite and selenate than wildtype plants.Citation1,Citation7 Conversely, treatment of MeJA or 1-aminocyclopropane-1-carboxylic acid (ACC; precursor of ethylene) enhanced selenite resistance in a Se-sensitive Arabidopsis ecotype, Ws-2.Citation1 These results further suggest that ethylene and JA are important for Se resistance in plants.
In selenite-treated Arabidopsis, we also observed accumulation of salicylic acid (SA).Citation1 This plant hormone is a major phenylpropanoid compound whose biosynthesis is triggered by various biotic and abiotic stresses.Citation11,Citation12 Although SA levels were enhanced by selenite, our results suggest that SA production inhibits acquisition of plant Se resistance rather than enhancing it, like JA and ethylene. Treatment with SA increased selenite sensitivity in a Se-resistant Arabidopsis accession, Col-0.Citation1 The underlying mechanism for this negative effect of SA on Se resistance is still unclear, but one possible explanation is crosstalk between ethylene, JA and SA pathways. Many studies have shown that these hormones act in mutually antagonistic or coordinated ways in plants suffering biotic or abiotic stresses. For example, it was shown that JA accumulation is prevented by the NPR1-dependent SA-signaling pathway in plants infected by Pseudomonas.Citation13 Also, SA is known to inhibit the activity of the last step in the ethylene biosynthesis pathway, ACC oxidase.Citation14 Thus, the observed increased Se sensitivity in the presence of SA might act through inhibition of ethylene and/or JA signaling pathways.
As described above, it appears that ethylene and JA play important roles in Se resistance in plants. Previous studies have shown that generation of reactive oxygen species (ROS) often preceeds ethylene and JA production.Citation15,Citation16 Our recent work showed that ROS production is enhanced in the Se-resistant accession Col-0.Citation1 On the other hand, very high levels of ROS production decreased Se-resistance in plants. Such plants showed signs of high levels of SA production, which may have attenuated ethylene and/or JA function as described above. Thus, it appears that optimal levels of ROS production are necessary for acquisition of Se-resistance in Arabidopsis. The source of ROS in Se-treated plants is still unclear and will require further study. Incidentally, ROS production was also observed in a selenite-treated cell suspension of coffee.Citation17
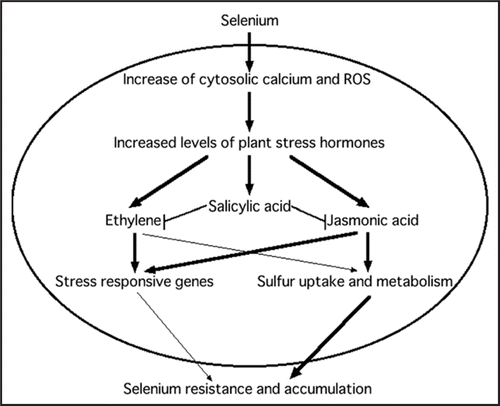
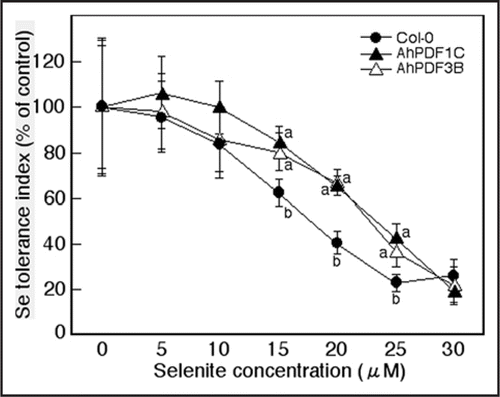
We would like to propose a simple model based on the results described in our recent papers (). According to our model, absorbed selenate or selenite generates ROS in the plant. Incidentally, an increase in cytosolic calcium concentration is also expected in these plants since many genes related to calcium signaling, such as calcium transporters, calcium binding proteins and calmodulin genes, were identified in the transcriptome analysis of selenate-treated plants.Citation7 The Se-induced ROS mimic an oxidative burst in plant cells, and the perception of this change triggers a wide array of signaling cascades similar to those induced by plant pathogens. Furthermore, Se-induced changes in the levels of free calcium may phosphorylate one of the subunits of NADPH-oxidase known to generate ROS, or it may directly affect NADPH oxidase activity because this enzyme has an N-terminal sequence with two calcium-binding EF-hand motifs.Citation18,Citation19 The generated ROS activate the production of ethylene, JA and SA. JA signaling upregulates both stress responsiveCitation13 and S uptake/metabolism genes.Citation9 Ethylene signaling also upregulates stress responsive genes, but the contribution of these genes to acquisition of Se resistance in plants is unclear. In this context, it is noteworthy that transgenic Arabidopsis thaliana that overproduce Arabidopsis halleri plant defensin (AhPDF1.1)Citation20 showed a slight but significant increase in tolerance to selenite compared to wild-type plants (). This same gene was shown earlier to confer Zn tolerance when overexpressed in Arabidopsis.Citation20 As the function of PDF proteins is still largely unknown, the mechanism of these positive effects of PDF1.1 on Zn and Se tolerance is unclear. Apart from possible functions of defense-related genes in the acquisition of Se resistance, the upregulation of S uptake/metabolism genes by JA likely is quite important for Se resistance. Several transgenic plants with enhanced Se accumulation and resistance have already been developed through overexpression of genes involved in S metabolism.Citation21–Citation23 Higher plant S levels likely help prevent incorporation of Se into S compounds, particularly proteins. Moreover, higher levels of the reduced S compound glutathione may help alleviate Se-induced oxidative stress. Any involvement of ethylene signaling in upregulation of S uptake/metabolism genes has not been shown until now. At low level, SA might have no effect on these processes, and thus on Se resistance, but at a higher level SA decreases plant Se resistance, perhaps through inhibition of ethylene and/or JA synthesis or signaling.
From an applied perspective, it is interesting that it appears from this study that increasing plant ethylene and/or JA levels may be a useful approach to develop plants with enhanced Se resistance and/or content. In this context it is interesting to note that providing plants with ethylene or JA precursor resulted in not only higher Se tolerance but also higher accumulation (unpublished results). From an evolutionary perspective, the finding that higher levels of ethylene and JA correlate with higher Se tolerance and accumulation may give insight into the evolution of Se hyperaccumulation. Indeed, a Se hyperaccumulator plant, Stanleya pinnata, (Brassicaceae) shows constitutive high levels of JA production (unpublished results). Production of plants with enhanced Se tolerance and accumulation will be useful both for producing Se-fortified food to avoid Se deficiency in humans and livestock, and to clean up excess Se from polluted soil and water.
Figures and Tables
Figure 1 Induction of AOS and PDF1.2 gene expression with selenite treatment. Seedlings of transgenic plants carrying a AOS promoter::GUS insert were grown without (A) or with (B) 15 µM selenite for 7 days, and stained with X-Gluc solution. Plants containing a PDF1.2 promoter::GUS insert were also grown without (C) or with (D) 15 µM selenite, and seedlings were GUS-stained.
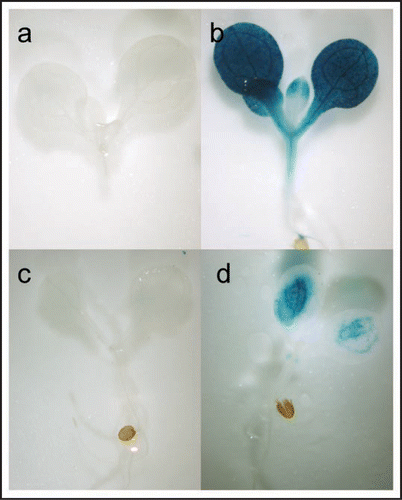
Figure 3 Selenite tolerance index for Col-0 (filled circles) and two lines of transgenic Arabidopsis thaliana expressing the Arabidopsis halleri PDF1.1 gene (AhPDF1C; filled triangles, AhPDF3B; open triangles). Plants were grown on control medium or on medium with various concentrations of sodium selenite for 10 days and measured for root length. Shown are the means ± SD (n = 20). Lower case letters indicate significant differences between Col-0 and transgenic plants for a particular selenite concentration (p < 0.05).
Acknowledgements
This work was supported by grants from the National Science Foundation (grant no. IOB-0444471 to E.P.S.) and The Ministry of Education, Science, Sports and Culture of Japan (grant no. 18780006 to M.T.).
Addendum to:
References
- Tamaoki M, Freeman JL, Pilon-Smits EAH. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis thaliana. Plant Physiol 2008; 146:1219 - 1230
- Wilber CG. Toxicology of selenium: a review. Clin Toxicol 1980; 17:171 - 230
- McNeal JM, Balisteri LS. Geochemistry and Occurrence of Selenium: An Overview. Selenium in Agriculture and the Environment 1989; Madison, WI Soil Science Society of America Special publication No. 23
- Hansen D, Duda P, Zayed AM, Terry N. Selenium removal by constructed wetlands: role of biological volatilization. Environ Sci Technol 1998; 32:591 - 597
- Terry N, Zayed AM, de Souza MP, Tarun AS. Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 2000; 51:401 - 432
- Stadtman TC. Selenium biochemistry. Ann Rev Biochem 1990; 59:111 - 127
- Van Hoewyk D, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smits EAH. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plant 2008; 132:236 - 253
- Stenzel I, Hause B, Miersch O, Kurz T, Mauzher H, Weichert H, Ziegler J, Feussner I, Wasternack C. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol Biol 2004; 51:895 - 911
- Sasaki Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Yokota Hirai M, Noji M, Saito K, Masuda T, Takamiya K, Shibara D, Ohta H. Coordinated activation of metabolic pathway for antioxidants and defence compounds by jasmonates and their roles in stress toletance in Arabidopsis. Plant J 2005; 44:653 - 668
- Penninckx IAMA, Thomma BPHJ, Buchla A, Métraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathway is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 1998; 10:2103 - 2114
- Durner J, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Trends Plant Sci 1997; 2:162 - 165
- Yalpani N, Enyedi AJ, León J, Raskin I. Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 1994; 193:372 - 376
- Lorenzo O, Solano R. Molecular players regulating the jasmonate signaling network. Curr Opin Plant Biol 2005; 8:532 - 540
- Leslie CA, Romani RJ. Inhibition of ethylene biosynthesis by salicylic acid. Plant Physiol 1988; 88:833 - 837
- Dong X SA. JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1998; 1:316 - 323
- Overmyer K, Brosch M, Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trend Plant Sci 2003; 8:335 - 342
- Gomes RA Jr, Gratão PL, Gaziola SA, Mazzafera PM, Lea PJ, Azevedo RA. Selenium-induced oxidative stress in coffee cell suspension cultures. Func Plant Biol 2007; 34:449 - 456
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homologue of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 1998; 10:255 - 266
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JD. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 1998; 14:365 - 370
- Mirouze M, Sels J, Richard O, Czernic P, Loubet S, Jacquier A, François IEJA, Cammue BPA, Lebrun M, Berthomieu P, Marquès L. A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J 2006; 47:329 - 342
- Pilon Smits EAH, Hwang S, Lytle CM, Zhu YL, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol 1999; 119:123 - 132
- Bañuelos G, Terry N, LeDuc DL, Pilon Smits EAH, Mackey B. Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environ Sci Technol 2005; 39:1771 - 1777
- Bañelos G, LeDuc DL, Pilon-Smits EAH, Terry N. Transgenic indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environ Sci Technol 2007; 41:599 - 605