Abstract
During the last two decades, we have analyzed the roles of boron (B) in the development of the legume-rhizobia symbiosis and nodule organogenesis. As in other plant tissues, B is needed for the maintenance of nodule cell wall structure. Moreover, several symbiotic events including rhizobial infection, nodule cell invasion and symbiosome development that involve membrane related functions (i.e. vesicle targeting, secretion, or cell surface interactions) are affected by B deficiency. Using anti-rhamnogalacturonan II (anti-RGII) antiserum and immunological techniques, we recently described membrane glycoproteins (RGII-glycoproteins) developmentally regulated in Pisum sativum nodules, which are not detected by the antibody in B-deficient nodules. RGII-glycoproteins appeared related with development processes involving extensive membrane synthesis, like symbiosome maturation or cell growth, both of them negatively affected by B deficiency. Here, we suggest that, besides maintaining cell wall structure, B is both stabilizing components of the membrane glycocalyx and promoting interactions between cell surfaces glycoconjugates that are important during the establishment of the symbiosis and during nodule development. Moreover, we hypothesize that B is playing a similar role during plant or animal embryogenesis and development.
Although boron requirement for plant growth was demonstrated in the early 1920s,Citation1 and numerous biochemical, physiological, and anatomical effects of B deficiency have been reported in plants, bacteria, and animals (reviewed in refs. Citation2 and Citation3), only two well defined functions of the micronutrient have been clearly demonstrated, both of them in the last decade: in plants, boron is maintaining cell wall structure by cross-linking apiose residues in the pectic polysaccharide rhamnogalacturonan II (RGII);Citation4 in bacteria of the genus Vibrio borate acts as a ligand in the cyclic furanosyl quorum-sensing signal AI-2.Citation5 Glycolipids of heterocystous cyanobacteria and hopanoids of bacteria of the genus Frankia are components of cell envelopes likely stabilized through borate cross-linking too.Citation6,Citation7 Therefore, formation of molecular bridges is a model for boron function that provides a single unifying explanation for such a diverse range of apparently unrelated biological roles,Citation8 and identifying boron-attracting molecules will shed new lights on this research topic. The effects of B deficiency not only in plant structure but also embryogenesis and development, together with the increasing evidence from animal and human experiments that boron is a trace element affecting an exceptionally large number of biological functions, situate the main role of B out of cell envelopes and point to borate-ligands, mainly glycoproteins, in cell membranes.
The symbiotic interaction between legume and rhizobia triggers the development of a nodule which encloses an interesting process of organogenesis and development.Citation9 Inside nodules bacteria proliferate and differentiate to N2-fixing bacteroids enclosed by a plant-derived peribacteroid membrane (PBM) which codifferentiate with bacteroids.Citation10 Nodule infected cells are full by several thousand of these organelle-like structures, termed symbiosomes. Consequently, an extensive synthesis and differentiation of membrane takes place at rates about 30- to 50-fold higher than in other tissues.Citation11 Therefore, legume nodule development provides an excellent model to investigate the role of B in membrane-located processes during differentiation, even although only a few cells are invaded in B-deficient nodules due to abortion of the infection process.Citation12
We have recently described glycoproteins reactive with anti-RGII antiserum (and therefore possible borate-ligands) that appear associated to the glycocalyx of the PBM of dividing symbiosomes but progressively disappear during symbiosome maturation.Citation13 Plasma membranes of pea (Pisum sativum) nodule and uninfected pea root cells also harbour RGII-glycoproteins but they were never detected in B-deficient cells suggesting that borate is stabilizing them in peribacteroid and plasma membranes. Symbiosomes develop aberrantly in the absence of B, appearing non N2-fixing undifferentiated bacteroids and PBM abnormally differentiated.Citation14 During symbiosome development, the carbohydrate moiety of PsNLEC 1, a lectin-like glycoprotein secreted to the peribacteroid fluid existing inside the symbiosome compartment, is associated both with the PBM and the bacterial cell surface.Citation15 This interaction seems to play a direct role for symbiosome development, since pea mutants lacking the symbiosomal form of PsNLEC 1 or cell surface defective rhizobia that do not interact physically with PsNLEC 1 do not develop N2-fixing bacteroids.Citation16,Citation17 Several authors link B with vesicle trafficking,Citation18,Citation19 and we demonstrated that, in the absence of RGII-glycoproteins due to B deficiency, the correct targeting and/or stabilization of vesicles containing new membrane or secreted material during symbiosome development, including PsNLEC 1 secretion, fails.Citation14
Taking into account those genetic and cytological evidences, we propose a model for a role of B on symbiosome development based on the stabilization of RGII-glycoproteins in the PBM and hence of plant-bacteria interactions (). In B-sufficient nodules, a differentiating PBM harbours RGII-glycoproteins and it is able to interact with PsNLEC 1. Glycoproteins stabilized by borate can either make possible the interaction or anchor directly to the symbiosomal nodule lectins. Once PsNLEC 1 is correctly located, a physical interaction with the bacterial cell surface, presumably through the O-oligosaccharide of the LPS (lipopolysaccharide),Citation15 drives bacteroid differentiation into a Y-shaped N2-fixing form. In the absence of B, the PBM do not have RGII-glycoproteins and association with PsNLEC 1 is either unstable or nonexistent. Therefore, PsNLEC 1 accumulates in the cytosol instead of the symbiosome compartment.Citation14 Communication between the bacteroid and the host cell, likely through a PsNLEC 1 mediated PBM-LPS bridge, does not work and bacteroids do not properly differentiate (as occurring in symbiotic associations involving pea mutants without targeting of PsNLEC 1 glycoproteins to the symbiosome or LPS-defective mutants of rhizobia).Citation16,Citation17
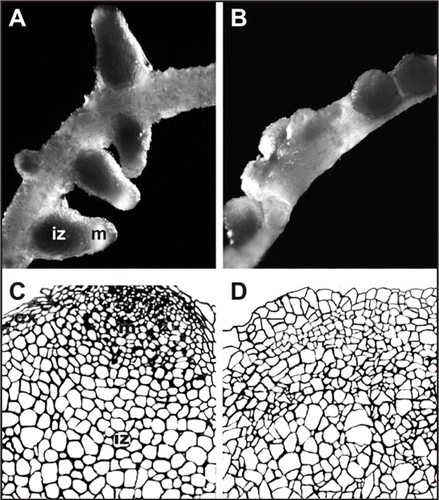
Besides PBMs, RGII-glycoproteins were never detected in plasma membranes of cells from B-deficient tissues, and, similar to that describe for symbiosome development, vesicle targeting during cell growth fails or becomes unstable. Therefore, not only symbiosome maturation, but also nodule organogenesis undergo abnormally in the absence of B (compare with ). Based on previously published anatomical studies,Citation20,Citation21 the sketch shown in and D illustrates nodule histology when it develops in the presence or in the absence of B respectively. Leaving aside infection and symbiosome development, B sufficient pea nodule anatomy ( and C) includes a persistent apical nodule meristem and a central zone of differentiated cells, most of them invaded by rhizobia (not drew) being other uninvaded interstitial cells conforming a network that communicate with vascular bundles. A highly differentiated nodule cortex completes the structure. During B-deficient nodule organogenesis there is not evident development of nodular tissues and cell proliferation without a proper differentiation process leads to tumour-like structures ( and D). Similar effects of B-deficiency have been described for animal organogenesis and for blastulation or gastrulation during vertebrate embryogenesis.Citation22–Citation24 Moreover, interesting new data indicate that boric acid inhibits proliferation of some human cancer cell lines.Citation25
Therefore, we propose that the model of stabilization by B of membrane glycoproteins involved in cell to cell communication can also apply during organogenesis and embryogenesis. Concerning nodule organogenesis, RGII-glycoproteins stabilized by B are apparently crucial for signalling during symbiosome, cell and tissue differentiation, and its description open the possibility that similar membrane components in animal cells play a similar role thanks to B.
Figures and Tables
Figure 1 Proposed model for a role of B on cell-surface interactions driving symbiosome development. Golgi derived vesicles (V) loaded with peribacteroid components, including lectin-like glycoprotein PsNLEC 1 (vc), are targeted to the symbiosome compartment. In the presence of borate anion (represented as B−) (+B panel), RGII-glycoproteins (GP) are stabilized on the inner face of vesicle membrane (VM). Then, it is able to interact with PsNLEC 1, which also anchors to the LPS of the bacteroid (B) reinforcing vesicle fusion. The triple association PBM-PsNLEC 1-bacteroid allows juvenile bacteroid differentiation into a mature N2-fixing form. In the absence of borate (−B panel), the vesicle membrane is not decorated with RGII-antigens; therefore it is unable to interact with PsNLEC 1. Consequently vesicle fusion becomes unstable and subsequent bacteroid development is arrested at a juvenile immature non N2-fixing stage.
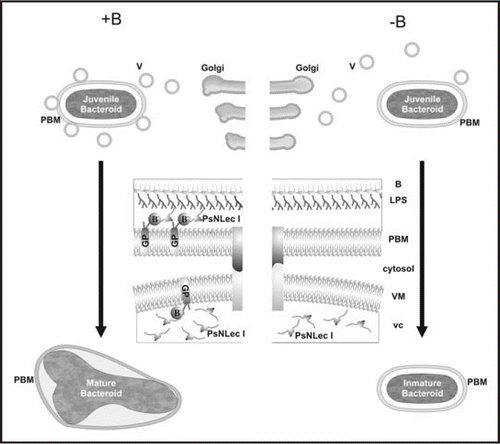
Figure 2 Effects of B on nodule organogenesis. (A) Pea root nodulated with sufficient B showing nodules with colourless apical nodule meristem (m) and dark central infected zones (iz). (B) Pea root nodulated in the absence of B, showing nodules with a tumour-like appearance when different development zones are undistinguishable. (C) Schematic illustration of B-sufficient nodule tissue organization indicating the apical meristem (m), the infected zone (iz), and the nodule cortex (cx). (D) Schematic illustration of highly disorganized B-deficient nodule structure with not clear development of different nodule tissues. (C and D) are based on (previously published studies, refs. Citation20 and Citation21).
Acknowledgments
This work was supported by Ministerio de Educación y Ciencia BIO2005-08691-CO2-01 and by MICROAMBIENTE-CM Program from Comunidad de Madrid. María Reguera is the recipient of a Contract from Comunidad de Madrid. Miguel Redondo-Nieto is the recipient of a Contract Juan de la Cierva.
Addendum to:
References
- Warington K. The effect of boric acid and borax on the broad been and certain other plants. Ann Bot 1923; 37:629 - 672
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Römheld V. Boron in plant biology. Plant Biol 2002; 4:205 - 223
- Xu F, Goldbac HE, Brown PH, Bell RW, Fujiwara T, Hunt CD, Goldberg S, Shi L. Advances in Plant and Animal Boron Nutrition 2007; Dordrecht Springer
- O'Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: Structure and function of a borate crosslinked cell wall pectic polysaccharide. Annu Rev Plant Biol 2004; 55:109 - 139
- Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler B, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002; 415:545 - 549
- García-González M, Mateo P, Bonilla I. Boron requirement for envelope structure and function in Anabaena PCC 7119 heterocysts. J Exp Botany 1991; 42:925 - 929
- Bolaños L, Redondo-Nieto M, Bonilla I, Wall LG. Boron requirement in the Discaria trinervis (Rhamnaceae) and Frankia symbiotic relationship. Its essentiality for Frankia BCU110501 growth and nitrogen fixation. Physiol Plant 2002; 115:563 - 570
- Bolaños L, Lukaszewski K, Bonilla I, Blevins D. Why boron?. Plant Physiol and Biochem 2004; 42:907 - 912
- Stougaard J. Regulators and regulation of legume root nodule development. Plant Physiol 2000; 24:531 - 540
- Perotto S, VandenBosch KA, Butcher GW, Brewin NJ. Molecular composition and development of the plant glycocalyx associated with the peribacteroid membrane of pea root nodules. Development 1991; 112:763 - 773
- Robertson JG, Lyttleton P. Division of peribacteroid membranes in root nodules of white clover. J Cell Sci 1984; 69:147 - 157
- Bolaños L, Brewin NJ, Bonilla I. Effects of boron on Rhizobium-legume cell-surface interactions and nodule development. Plant Physiology 1996; 110:1249 - 1256
- Redondo-Nieto M, Pulido L, Reguera M, Bonilla I, Bolaños L. Developmentally regulated membrane glycoproteins sharing antigenicity with rhamnogalacturonan II are not detected in nodulated boron deficient Pisum sativum. Plant Cell Environm 2007; 30:1436 - 1443
- Bolaños L, Cebrián A, Redondo-Nieto M, Rivilla R, Bonilla I. Lectin-like glycoprotein PsNLEC-1 is not correctly glycosylated and targeted in boron deficient pea nodules. Mol Plant-Microbe Interact 2001; 14:663 - 670
- Bolaños L, Redondo-Nieto M, Rivilla R, Brewin NJ, Bonilla I. Cell surface interactions of Rhizobium and other bacterial strains with symbiosomal and peribacteroid membrane components from pea nodules. Mol Plant-Microbe Interact 2004; 17:216 - 223
- Sherrier DJ, Borisov AY, Tikhonovich IA, Brewin NJ. Immunocytological evidence for abnormal symbiosome development in nodules of the pea mutant line Sprint2Fix− (sym31). Protoplasma 1997; 199:57 - 68
- Perotto S, Brewin NJ, Kannenberg EL. Cytological evidence for a host defense response that reduces cell and tissue invasion in pea nodules by lipopolysaccharide-defective mutants of Rhizobium leguminosartim strain 3841. Mol Plant-Microbe Interact 1994; 7:99 - 112
- Goldbach HE. A critical review of current hypotheses concerning the role of boron in higher plants: Suggestion for further research and its methodological requirements. J Trace Microprobe Tech 1997; 15:51 - 92
- Yu Q, Hlavacka A, Matoh T, Volkmann D, Menzel D, Goldbach HE, Baluska F. Short-term boron deprivation inhibits endocytosis of cell wall pectins in meristematic cells of maize and wheat root apices. Plant Physiol 2002; 130:415 - 421
- Bonilla I, Mergold-Villaseñor C, Campos ME, Sánchez N, Pérez H, López L, Castrejón L, Sánchez F, Cassab GI. The aberrant cell walls of boron-deficient bean root nodules have no covalently bound hydroxyprolin-/proline-rich proteins. Plant Physiol 1997; 115:1329 - 1340
- Redondo-Nieto M, Wilmot A, El-Hamdaoui A, Bonilla I, Bolaños L. Relationship between boron and calcium in the N2-fixing legume-rhizobia symbiosis. Plant Cell Environm 2003; 26:1905 - 1915
- Eckhert CD. Boron stimulates embryonic trout growth. J Nutr 1998; 128:2488 - 2493
- Fort DJ, Stover EL, Strong PL, Murray FJ, Keen CL. Chronic feeding of a low boron diet adversely affects reproduction and development in Xenopus laevis. J Nutr 1999; 129:2055 - 2060
- Rowe RI, Eckhert CD. Boron is required for zebrafish embryogenesis. J Exp Biol 1999; 202:1649 - 1654
- Meacham S, Elwell KE, Ziegler S, Carper SW. Xu F, Goldbac HE, Brown PH, Bell RW, Fujiwara T, Hunt CD, Goldberg S, Shi L. Boric acid inhibits cell growth in breast and prostate cancer cell line. Advances in Plant and Animal Boron Nutrition 2007; Dordrecht Springer 299 - 306